Figures & data
Figure 1. Cave hibernating Myotis lucifugus with white-nose syndrome caused by Pseudogymnoascus destructions. (a) White patches seen grossly on the muzzle, ear, and wing membrane (white arrows) are aerial hyphae with conidia. Photograph used with permission from Gregory Turner, Pennsylvania game commission, USA. (b) Dark areas of contraction (white arrow) are due to the infection of wing membrane with P. destructans. The black arrow points to a region of more normal tissue of the wing membrane. (c) A bat wing illuminated by 385-nm ultraviolet light. The yellow fluorescence corresponds to intraepidermal aggregates of hyphae [Citation4]. (d) Periodic acid Schiff-stained section of wing membrane from a bat with white-nose syndrome. Packets of intraepidermal, magenta-stained hyphae of P. destructans (arrows), previously termed ‘cupping erosions’ [Citation5], cause the fluorescence in (c). Absence of inflammatory cell reaction is consistent with immune downregulation that occurs during hibernation.
![Figure 1. Cave hibernating Myotis lucifugus with white-nose syndrome caused by Pseudogymnoascus destructions. (a) White patches seen grossly on the muzzle, ear, and wing membrane (white arrows) are aerial hyphae with conidia. Photograph used with permission from Gregory Turner, Pennsylvania game commission, USA. (b) Dark areas of contraction (white arrow) are due to the infection of wing membrane with P. destructans. The black arrow points to a region of more normal tissue of the wing membrane. (c) A bat wing illuminated by 385-nm ultraviolet light. The yellow fluorescence corresponds to intraepidermal aggregates of hyphae [Citation4]. (d) Periodic acid Schiff-stained section of wing membrane from a bat with white-nose syndrome. Packets of intraepidermal, magenta-stained hyphae of P. destructans (arrows), previously termed ‘cupping erosions’ [Citation5], cause the fluorescence in (c). Absence of inflammatory cell reaction is consistent with immune downregulation that occurs during hibernation.](/cms/asset/5075e353-71d1-4c0f-823a-af34c4efd57a/kvir_a_2082139_f0001_oc.jpg)
Figure 2. Wing membrane. Histologic periodic acid Schiff stain (a, b, d) and transmission electron micrographic (TEM, c) sections of wing membrane from Myotis lucifugus infected with Pseudogymnoascus destructans causing white-nose syndrome. Fungal hyphae are stained magenta. Lack of inflammatory cell reaction is consistent with WNS in hibernating bats [Citation5]. (a) Pigmented cells of the epidermal layer (arrowheads) illustrate the intraepidermal boundary above and below dense packets of irregular P. destructans hyphae without epidermal damage [Citation21]; this is consistent with the biotrophic stage of invasion. (b) Intraepidermal proliferation of fungal hyphae demarcated above and below by pigmented epidermis (arrowheads). Arrows show hyphae penetrating the basement membrane of the epidermis initiating infection of the deeper dermis. This hyphal invasion resembles the penetration mechanism used by some types of appressoria. (c) TEM shows fungal hyphae (arrows) within the outer layer (stratum corneum) of the epidermis. Arrowheads indicate the layer of bat epidermal cells covering hyphae without morphologic change or evidence of damage to the host cells; this is consistent with biotrophic infection. (d) Progression of P. destructans infection into deeper dermis (arrows) with replacement of connective tissue of the wing membrane with fungal hyphae.
![Figure 2. Wing membrane. Histologic periodic acid Schiff stain (a, b, d) and transmission electron micrographic (TEM, c) sections of wing membrane from Myotis lucifugus infected with Pseudogymnoascus destructans causing white-nose syndrome. Fungal hyphae are stained magenta. Lack of inflammatory cell reaction is consistent with WNS in hibernating bats [Citation5]. (a) Pigmented cells of the epidermal layer (arrowheads) illustrate the intraepidermal boundary above and below dense packets of irregular P. destructans hyphae without epidermal damage [Citation21]; this is consistent with the biotrophic stage of invasion. (b) Intraepidermal proliferation of fungal hyphae demarcated above and below by pigmented epidermis (arrowheads). Arrows show hyphae penetrating the basement membrane of the epidermis initiating infection of the deeper dermis. This hyphal invasion resembles the penetration mechanism used by some types of appressoria. (c) TEM shows fungal hyphae (arrows) within the outer layer (stratum corneum) of the epidermis. Arrowheads indicate the layer of bat epidermal cells covering hyphae without morphologic change or evidence of damage to the host cells; this is consistent with biotrophic infection. (d) Progression of P. destructans infection into deeper dermis (arrows) with replacement of connective tissue of the wing membrane with fungal hyphae.](/cms/asset/911840c4-0e2b-4959-9e10-c2c9d4978f3a/kvir_a_2082139_f0002_oc.jpg)
Figure 3. Muzzle. Histologic sections of muzzle from Myotis lucifugus stained with hematoxylin and eosin (a), periodic acid Schiff stain (b, d), and transmission electron micrographic (TEM, c). (a) Normal muzzle tissue stained with hematoxylin and eosin. Arrowhead points to hair shaft. Black arrow points to sebaceous gland that secretes lipid material into the hair follicle. (b) Fungal hyphae of Pseudogymnoascus destructans (magenta) within and expanding from the follicle (arrowheads), invading and replacing lipid-laden sebaceous gland (arrow) and surrounding connective tissue. (c) TEM shows fungal hyphae (black arrows) entering the hair follicle identified by emerging hair shaft (white arrow). (d) Extensive invasion of P. destructans including hair follicle surrounding hair shaft (arrowheads) with replacement of associated sebaceous glands (arrows) and regional connective tissue.
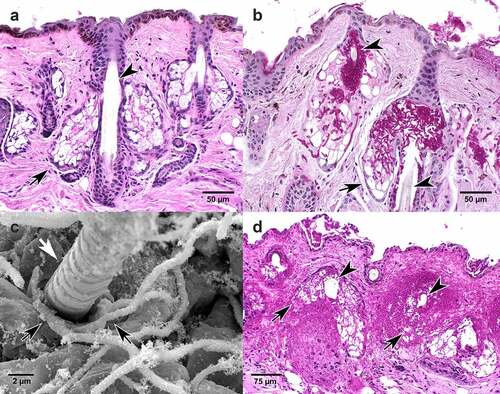
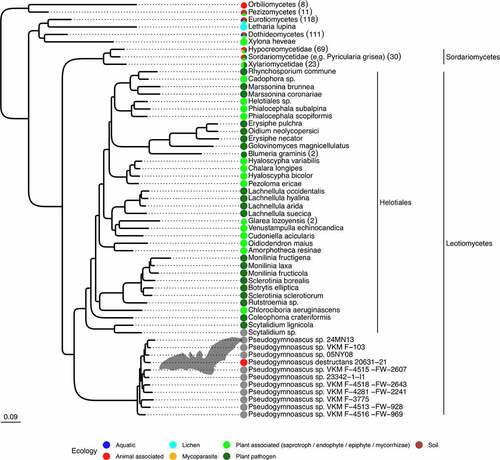
Figure 4. Pseudogymnoascus species are phylogenetically related to plant-associated fungi. Maximum likelihood tree based on 1,146 families of orthologues. All displayed nodes are supported by a bootstrap value >95%. The number of sequences in each collapsed clade are indicated within parentheses. The tree is represented in its more complete form inFigure S-1. Within the Leotiomyceta clade, groups outside Pseudogymnoascus have been collapsed at the species level, with the corresponding number of strains indicated in parentheses. Color dots indicate known ecologies. The Leotiomyceta clade does not contain any representatives of the ecology groups for aquatic, lichen, mycoparasite of plant pathogen, or mammalian pathogens outside of the Pseudogymnoascus genus. Additional species have been discarded because they were closely related to species with more sequence data, a list of which can be found in Supplementary Table S1.
Supplemental Material
Download Zip (690 KB)Data availability statement
The data that support the findings of this study are openly available in Zenodo at DOI reference number 10.5281/zenodo. 5939906 at https://zenodo.org/record/5939906#.YjpiN-rMKUk.
