Figures & data
Figure 1. Graphical Abstract: Testing recombination and complementation during coinfection in vivo. (a) Chickens were inoculated with a mixture of MDV and vacMD to create a coinfection scenario. (b) Inoculated chickens were housed with uninfected contact chickens. (c) Approximately 2 weeks post-inoculation, chickens infected with viruses are positive in their feathers that was visualized using fluorescent stereomicroscopy. (d) in Experiment 1, five contact chickens were positive for red vacMD, while one chicken (bird #9620) was positive for green MDV based on fluorescence. In Experiment 2, no contact chickens were positive for red vacMD, while one contact chicken (bird #9628) was positive for green MDV. PCR on DNA extracted from infected feathers of birds #9620 and #9628 using MDV specific primers for gC. DNA from the inoculum (MDV-NT) and wild-type MDV (MDV-T) were used as positive controls in which MDV-NT generates a 422 bp product, while MDV-T produces full-length 1,925 bp product encoding MDV gC. PCR for MDV UL13 was used as an internal control for MDV genomes and DNA quality. (e) Viruses isolated from tumors obtained from bird #9620 (v9620) and #9628 (v9628) were propagated for two passages and DNA was used in PCR assays, as was done for DNA from feathers to confirm they remained gC negative. (f) v9620 and v9628 isolated in (E) were inoculated into chickens and chickens were monitored for replication in FFs and MD induction. Contact chickens were housed with v9620- and v9628-infected chickens and remained negative for MDV over 8 weeks confirming both viruses did not gain transmissibility through a genetic component. .
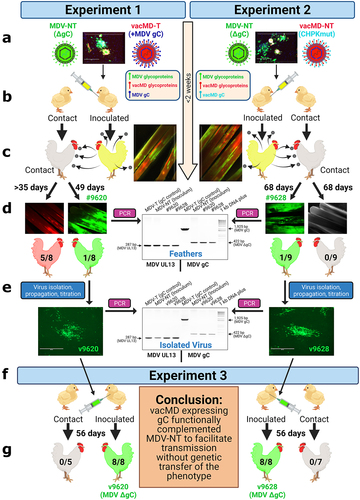
Figure 2. Coinfection of MDV-NT and vacMD in CEC cultures. (a) Schematic representation of transmissible (T) and non-transmissible (NT) MDV and vacMD used in this study. MDV-NT lacks gC (MDV δgc) that is required for transmission [Citation8,Citation9,Citation23]. vacMD was rendered NT through mutation of its CHPK activity by mutation of the invariant lysine (K157A) that is required for transmission [Citation18]. vacMD-T expresses MDV gC in place of its gC [Citation17]. (b) Following inoculation into chickens, the inoculum was back tittered in CEC cultures and shown are representative plaques for MDV-NT and vacMD-NT (vacMD-T not shown). Direct visualization of pUl47mrfp was used to identify vacMD-NT plaques, as indicated by strong nuclear expression as previously shown [Citation17], while expression of MDV pUl47egfp is barely detectable in cell culture [Citation26]; therefore, polyclonal anti-MDV antibody was used to identify MDV-NT plaques with anti-chicken IgY secondary antibody conjugated with AlexaFluor488. Anti-MDV pAb has cross reactivity with homologous vacMD and thus stains both MDV- and vacMD-induced plaques but stains MDV plaques with greater intensity. The intense staining with pAb and negative for pUl47mrfp expression indicates MDV infected plaques, while light staining with pAb and high pUl47mrfp nuclear expression is indicative of vacMD infection.
![Figure 2. Coinfection of MDV-NT and vacMD in CEC cultures. (a) Schematic representation of transmissible (T) and non-transmissible (NT) MDV and vacMD used in this study. MDV-NT lacks gC (MDV δgc) that is required for transmission [Citation8,Citation9,Citation23]. vacMD was rendered NT through mutation of its CHPK activity by mutation of the invariant lysine (K157A) that is required for transmission [Citation18]. vacMD-T expresses MDV gC in place of its gC [Citation17]. (b) Following inoculation into chickens, the inoculum was back tittered in CEC cultures and shown are representative plaques for MDV-NT and vacMD-NT (vacMD-T not shown). Direct visualization of pUl47mrfp was used to identify vacMD-NT plaques, as indicated by strong nuclear expression as previously shown [Citation17], while expression of MDV pUl47egfp is barely detectable in cell culture [Citation26]; therefore, polyclonal anti-MDV antibody was used to identify MDV-NT plaques with anti-chicken IgY secondary antibody conjugated with AlexaFluor488. Anti-MDV pAb has cross reactivity with homologous vacMD and thus stains both MDV- and vacMD-induced plaques but stains MDV plaques with greater intensity. The intense staining with pAb and negative for pUl47mrfp expression indicates MDV infected plaques, while light staining with pAb and high pUl47mrfp nuclear expression is indicative of vacMD infection.](/cms/asset/40f9cb43-9eb9-40d2-ada6-0b014187bef0/kvir_a_2082645_f0002_oc.jpg)
Figure 3. Superinfection inhibition and coinfection of MDV and vacMD in feathers. (a) Coinfected feathers from experimentally infected chickens. Arrowheads (>) indicate coinfected regions where both green MDV and red vacMD are evident in the merged image creating yellow, while asterisks (*) indicate regions where there is a clear delineation of green MDV and red vacMD in proximity suggestive of superinfection inhibition. (b) a representative cryosection of green MDV and red vacMD coinfected FFE skin cells suggestive of superinfection inhibition. The region highlighted with a white square is shown at greater magnification below. Clear delineation of green MDV and red vacMD can be seen and highlighted with dotted line and asterisk (*). (c) a representative cryosection of green MDV and red vacMD coinfected FFE skin cells. Regions 1 and 2 are magnified below and coinfection is clearly observed, indicated by merging of green MDV and red vacMD to generate yellow cells. Nuclei were stained with Hoechst 33342 to better visual cellular structure. Feather follicles (FF) and FF epithelial (FFE) skin cells are labeled in the images with arrows (→).
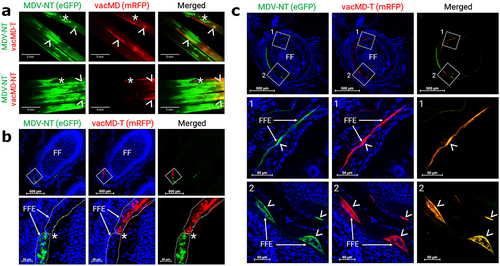
Figure 4. MDV gC expression in coinfected FFs and dual infected FFE skin cells. (a) Cryosection of FFs showing both green MDV and red vacMD individually infecting FFE skin cells. Anti-MDV gC antibody was used to identify MDV gC positive cells. Only vacMD-T showed staining, while green MDV-NT-infected cells were negative. (b) Cryosection of an FF where dual infection with green MDV and vacMD was clear. Anti-MDV antibody stains red vacMD-infected cells, including dual infected “yellow” cells. Feather follicles (FF) and FF epithelial (FFE) skin cells are labeled in the images with arrows (→) and arrowheads (>) indicate MDV gC staining. Nuclei were stained with Hoechst 33342 to better visual cellular structure.
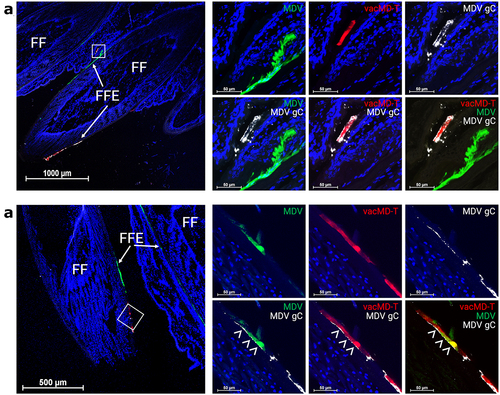
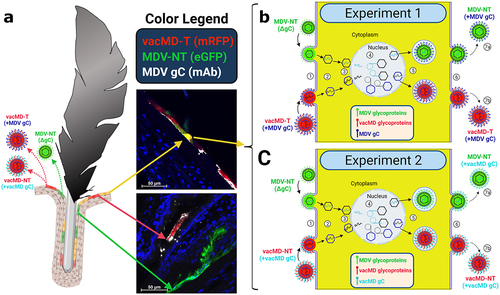
Figure 5. Cartoon depiction of functional complementation in vivo. (A) Coinfection of chickens with MDV and vacMD results in FFE skin cells individually infected with each virus (shown as red or green), as well as coinfection in which both viruses are present (shown as yellow). Through normal virus replication in cells, red and green cells would produce progeny of the infecting virus and thus, red cells produce vacMD-T or -NT, while green cells produce MDV-NT. Representative images of red, green, and yellow cells are shown, including staining for MDV gC with mAb (white). (B and C) Basic replication cycle of herpesviruses including (1) entry into cells, (2) uncoating of tegument, (3) docking of nucleocapsid to the nuclear membrane and expulsion of genomic DNA into the nucleus. In the nucleus, (4) viral DNA is duplicated and encapsulated into naked capsids to produce newly generated nucleocapsids that then exit the nucleus (5) where tegumentation and primary envelopment produces virions that egress (6) from the cell as cell-free virus. In dually infected cells, an infinite combination of viral proteins and genomes can be produced if compatible (not shown). Based on the results presented in this report, MDV-NT genomes were packaged into virions in which gC provided from vacMD-T (+MDV gC) or vacMD-NT (+vacMD gC) to generateinfectious MDV able to naturally infect chickens (7a), while the original vacMD was also produced (7b) in each experiment. Thus, transmissible MDV-NT virions would be produced, disseminated into the environment, and naturally infected naïve contact chickens. However, infected contact chickens would be unable to produce transmissible MDV-NT, as shown in Experiment 3, without complementation from coinfection with vacMD.
Supplemental Material
Download Zip (2.8 MB)Data availability statement
The authors confirm that the data supporting the findings of this study are available with the article and its supplementary materials.
