Figures & data
Table 1. Natural history of HBV infection divided into 5 phases. +++++: almost always detected; ++++: frequently detected; +++: often detected; ++: occasionally detected; +: rarely detected.
Table 2. Definition of HBV cure. ± Anti-HBsAg may be detected.
Table 3. Advantages and disadvantages of NAs and PEG-IFN-α in the treatment of CHB.
Figure 1. HBV life cycle and novel therapeutic targets. DAAs, HTT and cccDNA direct-acting agents are shown orange, red, and green, respectively. ASO: antisense oligonucleotides; cccDNA: covalently closed circular DNA; ER: endoplasmic reticulum; dslDna: double-stranded linear DNA; FXR: Farnesoid X receptor; NTCP: sodium-taurocholate co-transporting polypeptide; MVB: multivesicular bodies pcRNA: precore RNA; pgRNA: pregenomic RNA; Pol: polymerase; rcDNA: relaxed circular DNA, siRNA: small interfering RNA; SVP: subviral particles.
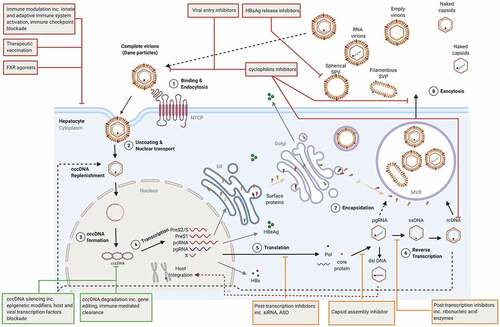
Table 4. Summary of novel antiviral therapies for CHB in clinical trial: (a) Single therapies; (b) Combination therapies. CI: Capsid inhibitor; Cyp I: cyclophilins inhibitors; HF: Host factors; Janssen Research & dvp: Janssen Research & development; Nivo: Nivolumab; NMAb: Neutralising monoclonal antibodies; NAPs: nucleic acid polymers; mAb: monoclonal antibodies; PEG: PEG-IFN-α; Pharm.: Pharmaceutical; TV: therapeutic vaccines; TLRa: TLR agonists; VIR Biotech: VIR Biotechnology. Clinical trials in the recruitment, active or not yet in the recruitment phase are highlighted in blue, green and red respectively. completed trials are excluded.
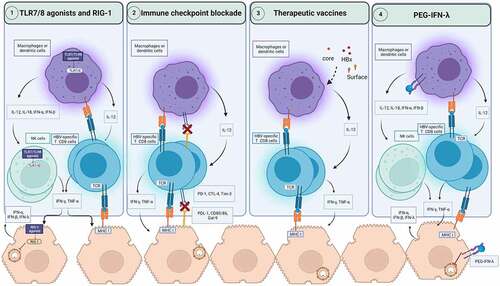
Figure 2. Immune based therapeutic strategies for CHB. The activation of pathogens recognition receptors such as TLR7, TLR8 and RIG-1 by agonists can induce the secretion of antiviral cytokines by hepatocytes and innate immune cells such as macrophages, dendritic cells, and NK cells. This activation of innate immunity can restore HBV-specific T cells to produce antiviral cytokines such as IFN-γ and TNF-α. RIG-1 can also exerts direct antiviral activities against HBV. Immune checkpoint blockade prevents the engagement of inhibitory checkpoints express on HBV-specific T cells with their ligands on macrophages, dendritic cells, and hepatocytes. As a result, HBV-specific T cells functionality can be restored to produce antiviral cytokines and potentially cytotoxic response to clear infected hepatocytes. Therapeutic vaccines encoding for viral antigens, can enhance cross-presentation of these antigens and boost HBV-specific T cell response. PEG-IFN-λ therapy can modulate dendritic cells, and macrophages and mediate the cross talk with NK and HBV-specific T cells. In hepatocytes, PEG-IFN-λ induces the engagement of signaling pathways which suppress HBV replication. CTLA-4: Cytotoxic T-lymphocyte associated protein 4; IFN: Interferon; IL: Interleukin; MHC: Major histocompatibility complex; NK cells: natural killer cells; PEG-IFN-λ: Pegylated interferon-lambda; PD-1: Programmed death pathway-1; RIG-1: Retinoic acid-inducible gene; Tim-3: T cell immunoglobulin and mucin-domain containing-3; TLR: Toll-like receptor; TNF-α: Tumor necrosis factor-alpha.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.
