Figures & data
Figure 1. M. pneumoniae induces secretion of MMP-9 from bronchial epithelial cells. BEAS-2B cells or primary bronchial epithelial cells were infected with M. pneumoniae at a multiplicity of infection (MOI) of 0, 5, 25, and 100 for 16 h, or at an MOI of 100 for 8–24 h. The mRNA and protein expression levels were determined by qPCR and immunoblotting (a–d, f–i), and the enzymatic activity of MMP-9 in the supernatant was measured by gelatin zymography (e, j). Representative results from three independent experiments are shown. *P < 0.05, compared with the control group (0 MOI or 0 h).
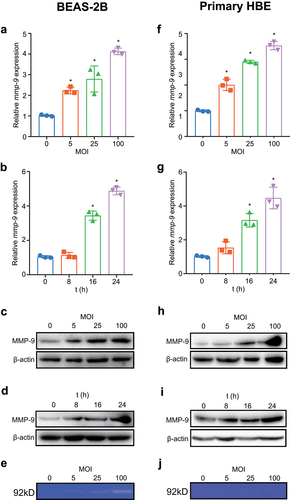
Figure 2. Inhibition of TLR2/6 shows reduced MMP-9 expression upon M. pneumoniae infection. BEAS-2B cells were plated in a 6-well plate, and TLR inhibition experiments were performed by transfection of 0.5 μg of empty vector (pZERO), DN-TLR1, DN-TLR2, or DN-TLR6 plasmid for 20 h before infection with M. pneumoniae (MOI 1:100) for another 16 or 24 h. Total RNA or protein was assayed for MMP-9 expression by qPCR (a–c) or immunoblotting (d–f). Experiments were repeated three times, and representative data are shown. *P < 0.05, compared with the indicated groups.
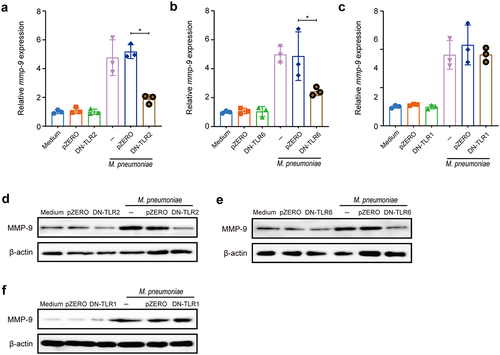
Figure 3. M. pneumoniae activates MAPKs to modulate MMP-9 expression. BEAS-2B cells were infected with 100 MOI of M. pneumoniae for 1 h and then phosphorylated, and total ERK1/2, JNK1/2, and p38 levels were detected by immunoblotting (a). For inhibitor experiments, cells were pretreated with 30 μM of MAPK inhibitors U0126 (ERK), SP600125 (JNK), or SB203580 (p38) for 30 min, and after 16 or 24 h of M. pneumoniae infection, MMP-9 mRNA (b–d) and protein (e–g) levels were detected by qPCR and immunoblotting, respectively. The experiment was repeated three times, and representative data are shown. *P < 0.05, compared with the control group.
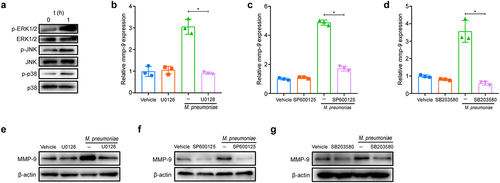
Figure 4. M. pneumoniae-stimulated MMP-9 expression requires MAPK/NF-κB. BEAS-2B cells were stimulated with 100 MOI of M. pneumoniae for 0, 0.5, 1, and 2 h. Then, IκBα expression was measured by immunoblotting (a). Cells were pretreated with or without MAPK inhibitors for 30 min before infection with M. pneumoniae for 1 h. Activation of NF-κB was assessed by immunofluorescence (b). BEAS-2B cells were preincubated with 5 μM BAY11–7082 for 30 min. After 16 h of infection, MMP-9 mRNA expression was detected using qPCR (c,d). BEAS-2B cells were pretreated with MAPK inhibitors for 30 min before M. pneumoniae infection. IκBα expression was detected by immunoblotting (e–g). The results were obtained from three independent experiments performed in triplicate. *P < 0.05, compared with the indicated groups.
Figure 5. Requirement of AP-1 for efficient induction of MMP-9. Cells were seeded and infected with M. pneumoniae for 1 h. Phosphorylation of c-fos and c-jun was assessed by immunoblotting with or without specific inhibitors (MAPK inhibitors, 30μM and BAY11–7082, 5μM) (a–d). BEAS-2B cells were pre-incubated with or without inhibitors prior to M. pneumoniae infection. AP-1 recruitment to the MMP-9 binding site was detected using a ChIP assay (e–f). Representative results from three independent experiments are shown.
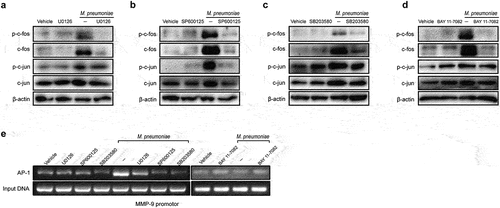
Figure 6. Histone acetylation is required for M. pneumoniae-induced MMP-9 expression. Cells were seeded and infected with M. pneumoniae (MOI = 100) for 0–2 h, and the acetylation status of histones H3 and H4, as well as the expression of HDAC1 and HDAC2 in cells, were detected by immunoblotting (a,b). (c) Total HDAC activity in M. pneumoniae-infected cells was measured (c). BEAS-2B cells were pretreated with an HDAC inhibitor (TSA, 100 ng/ml) for 30 min before infection with M. pneumoniae for 16 h, and qPCR was used to detect MMP-9 mRNA expression (d). The results were obtained from three independent experiments performed in triplicate. *P < 0.05, compared with the indicated groups.
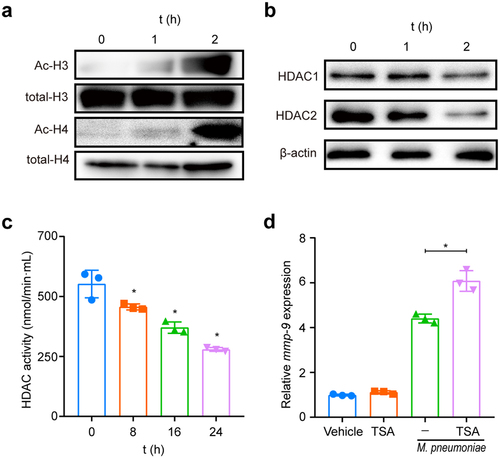
Figure 7. M. pneumoniae increases the MMP-9/TIMP-1 ratio. BEAS-2B cells were infected with M. pneumoniae (MOI = 0, 5, 25, and 100) for 16 or 24 h. TIMP-1 and MMP-9 expression was detected by qPCR and immunoblotting, and the MMP-9/TIMP-1 ratio was calculated (c). Representative results from three independent experiments are shown. *P < 0.05, compared with the control group (0 MOI).
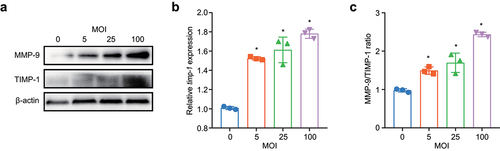
Figure 8. M. pneumoniae activates Sp1 to downregulate RECK expression. BEAS-2B cells were infected with different MOIs of M. pneumoniae for 16 h. RECK expression was measured using qPCR and immunoblotting (a,b), and the phosphorylation level of Sp1 was determined using immunoblotting (c). The cells were pretreated with an Sp1 inhibitor (mithramycin A; MA; 2 μM) for 30 min before being infected with M. pneumoniae for 16 h. The expression of RECK and MMP-9 was assessed using qPCR (d,e), and MMP-9 enzyme activity was determined by gelatin zymography (f). BEAS-2B cells were transfected with a RECK-overexpressing plasmid and then infected with M. pneumoniae for 16 h. MMP-9 expression and enzymatic activity were measured by qPCR, immunoblotting, and gelatin zymography (g–i). Results are from three independent experiments performed in triplicate, *P < 0.05, compared with the control group (0 h) or the indicated group.
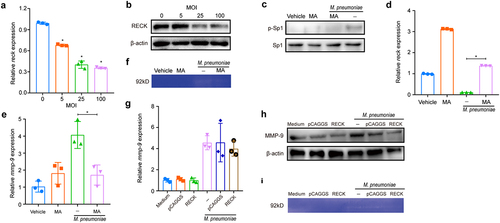
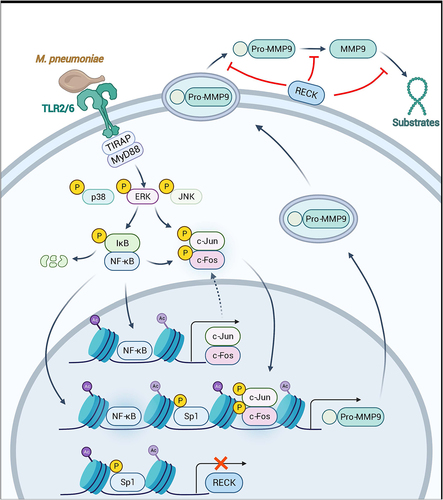
Figure 9. Schematic model depicting the potential signaling pathway involved in M. pneumoniae-induced MMP-9 secretion in bronchial epithelial cells. M. pneumoniae induces MMP-9 expression by activating the MAPK/NF-κB/AP-1 cascade, modulated by TLR2 and TLR6. M. pneumoniae induces the acetylation of histone and phosphorylation of Sp1 by unknown mechanisms. Histone acetylation facilitates MMP-9 expression, while Sp1 phosphorylation results in the downregulation of RECK. Decreased RECK expression weakened the inhibitory effect on MMP-9 release and enzymatic activity, thereby contributing to the secretion of active MMP-9 in the host (The figure is created with BioRender.Com).
Supplemental Material
Download MS Word (667.4 KB)Data availability statement
The data supporting the findings of this study are available from Xiaoxing You upon reasonable request.
