Figures & data
Figure 1. PEDV induces autophagy. Vero and IPEC-DQ cells were infected with the indicated MOI (0, 005, 0.05, and 0.5) of PEDV HXLV and HLJBY strains for 16 hr or 36, respectively (a), or with 0.5 MOI for the indicated time (b). (c & d) Vero and IPEC-DQ cells were mock-infected or infected with PEDV HXLV and HLJBY strains (0.5 MOI each), respectively. After incubation for 8 hr, bafilomycin a (Baf) (20 nM) or chloroquine (CQ) (10 µM) was added and incubated for another 8 hr. LC3 lipidation and the levels of p62, the viral nucleoprotein (N) and spike (S) proteins were analysed by Western blot. β-actin was detected as a loading control. *p < 0.05, **p < 0.01, compared to uninfected controls; #p < 0.05, ##p < 0.01, compared to PEDV-infected cells.
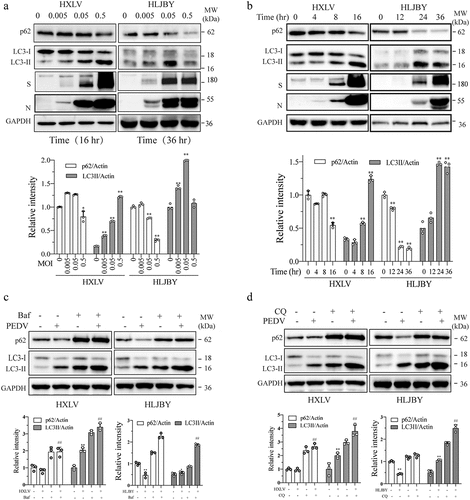
Figure 2. PEDV-Induced autophagy facilitates virus replication. (a) GFP-RFP-LC3-transfected Vero cells were mock-infected or infected with PEDV virus (0.5 MOI). After incubation for 8 hr., CQ (10 µM) and bafilomycin a (20 nM) were added and incubated for another 8 hr. After nuclei staining with DAPI, orange autophagosomal puncta and red autolysosomal puncta were examined and under a confocal microscope and quantified. Scale bar, 50 µm. The means of autolysosomes shown in red bars and autophagosomes shown in orange bars per cell were plotted in a bar graph (b). (c & d) Autophagy facilitates PEDV replication. Vero cells were inoculated with PEDV (0.5 MOI) and then incubated with or without bafilomycin a (20 nM) or CQ (10 µM) for 12 hr. Virus replication was examined by analysing the viral S and N protein levels with Western blot (c). Virus titres in the conditioned media were analysed by measuring the TCID50 values (d). The results represent the mean ± SD of four independent experiments. *p < 0.05; **p <0.01 compared to PEDV-infected cells. (e) Effect of bafilomycin a and CQ on Vero cell proliferation. Bafilomycin a (20 nM) and CQ (10 µM) were added into uninfected or PEDV HXLV strain-infected Vero cells and incubated for 12 hr. Cell proliferation was evaluated by using a CellTiter-Glo kit. The mean ± SD of the triplicate from a representative experiment was shown in a bar graph. The experiment was repeated twice with similar results. (f) Vero and IPEC-DQ cells were transfected with Beclin-1 and ATG5 siRNA, respectively. Transfection with a scrambled siRNA was included as a negative control. After incubation for 36 hr, Vero and IPEC-DQ cells were infected with HXLV and HLJBY virus, respectively. Twelve hours later, the levels of the S protein of PEDV and Beclin-1 or ATG5 were detected by Western blot.
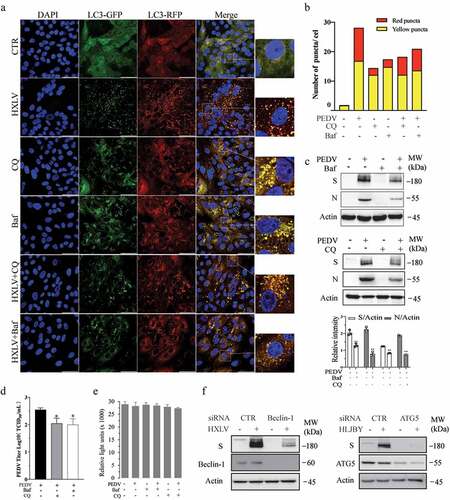
Figure 3. JNK activation contributes to PEDV-induced autophagy. (a) IPEC-DQ and Vero cells were infected with PEDV HLJBY and HXLV strains and incubated for 24 and 12 hr, respectively. JNK phosphorylation and total JNK protein levels were analysed by Western blot. (b) Vero cells inoculated with 0.5 MOI PEDV were incubated without or with SP600125 (SP) (10 µM) for 12 hr. The levels of LC3, p62, and JNK phosphorylation were analysed by Western blot. *p <0.05, compared to uninfected controls; #p < 0.05, ##p <0.01, compared to PEDV-infected cells. (c & d) GFP-RFP-LC3-transfected Vero cells were mock-infected or infected with PEDV HXLV virus (0.5 MOI) and incubated for 8 hr. SP600125 (10 µM) was then added and incubated for another 8 hr. After nuclei staining with DAPI, orange autophagosomal puncta and red autolysosomal puncta were examined and quantified under a confocal microscope (c). The mean numbers of autolysosomes shown in red bars and autophagosomes shown in orange bars per cell were plotted in a bar graph (d). (e & f)Vero cells infected with PEDV (0.5 MOI) were treated with dimethyl sulphoxide (DMSO 0.5%) or SP600125 (10 µM) for 12 hr. The S and N proteins in the cell lysates and conditioned media were analysed by Western blot (e). The levels of the S and N proteins relative to β-actin was analysed and plotted in a bar graph. The conditioned media of PEDV-infected cells were titrated for the TCID50 values (f). #p < 0.05, ##p < 0.01, compared to PEDV-infected cells. (g) SP600125 does not affect cell Viability. Uninfected and PEDV-infected Vero cells were treated with DMSO or SP600125 (10 µM) for 12 hr. Cell viability was analysed by using a CellTiter-Glo kit. The mean ± SD of the triplicate from a representative experiment was shown in a bar graph. The experiment was repeated twice with similar results.
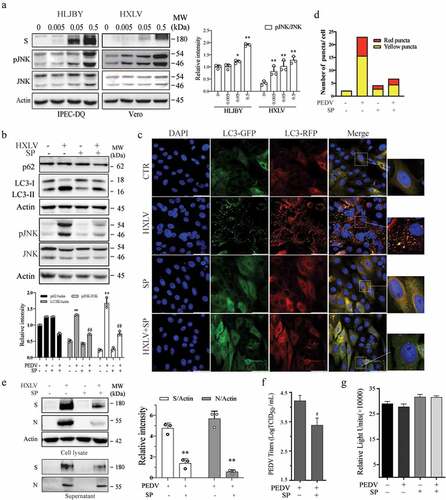
Figure 4. PEDV infection increases the levels of AMPK and ULK1 phosphorylation. Vero and IPEC-DQ cells were mock-infected or infected with HXLV and HLJBY viruses for 12 or 24 hr, respectively, or with 0.5 MOI of HXLV virus for the indicated lengths of time. AMPKT172 and ULK1S777 phosphorylation was detected by Western blot. *p <0.05, **p <0.01, compared to uninfected controls.
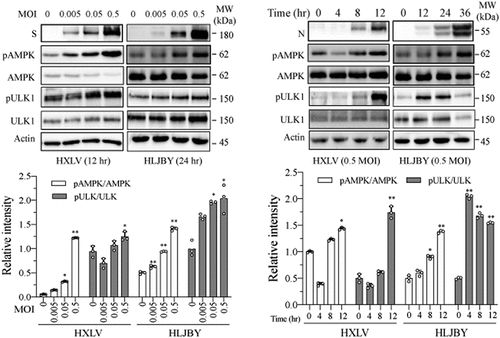
Figure 5. AMPK inhibition suppresses autophagy and PEDV replication. (a) Compound C (CC) suppresses autophagy in PEDV-infected cells. Mock- or HXLV virus-infected Vero cells were treated with DMSO (0.5%) or CC (2 µM) for 12 hr. LC3 lipidation, p62, and AMPKT172 and ULK1S777 phosphorylation were detected by Western blot. (b & c) CC suppresses autophagosome formation in PEDV-infected cells. GFP-RFP-LC3-transfected Vero cells were mock-infected or infected with HXLV virus (0.5 MOI). After incubation for 8 hr, the cells were treated with DMSO (0.5%) or CC (2 µM) for 8 hr. After nuclei staining with DAPI, orange autophagosomal puncta and red autolysosomal puncta were examined under a confocal microscope and quantified (b). The mean numbers of autolysosomes shown in red bars and autophagosomes shown in orange bars per cell were plotted in a bar graph (c). (d & e) CC inhibits virus growth. Mock or PEDV-infected Vero cells were treated with DMSO (0.5%) or CC (2 µM) for 12 hr. Viral proteins in the conditioned media and in cell lysates were analysed by Western blot (d). The virus titres in the conditioned media were analysed for the TCID50 values (e). *p < 0.05, **p < 0.01. (f) CC does not affect Vero cell proliferation. Uninfected and PEDV-infected Vero cells were incubated with DMSO (0.5%) or CC (2 µM) for 12 hr and then examined for cell viability by using a CellTiter-Glo kit. The data represents the mean ± SD of the triplicate from a representative experiment, which was repeated twice with similar results.
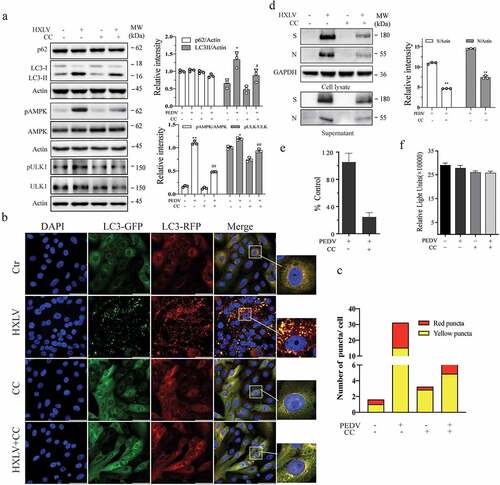
Figure 6. ULK1 activation contributes to PEDV-induced formation of autolysosomes and virus replication. (a) SBI -0,206,965 (SBI) inhibits PEDV-induced autophagy. Uninfected or PEDV-infected Vero cells were treated with DMSO (0.5%) or SBI (1 µM) for 12 hr. LC3 and p62 were detected by Western blot. (b & c) GFP-RFP-LC3-transfected Vero cells were mock-infected or infected with PEDV (0.5 MOI) and incubated for 8 hr. The cells were then treated with DMSO (0.5%) or SBI (1 µM) for another 8 hr. After nuclei staining with DAPI, orange autophagosomal puncta and red autolysosomal puncta were examined under a confocal microscope and quantified (b). The numbers of puncta were calculated and presented as a bar graph (c). (d)uninfected or PEDV-infected Vero cells were treated with DMSO (0.5%) or SBI (1 µM) for 12 hr. Viruses released into the conditioned media were titrated by measuring TCID50 values. Data are the mean ± SD of three independent experiments. (e) SBI does not affect cell viability. Uninfected or PEDV-infected Vero cells were treated with DMSO (0.5%) or SBI (1 µM). After incubation for 12 hr, cell viability was examined by using a CellTiter-Glo kit. Data represent the mean ± SD of the triplicate from a representative experiment, which was repeated twice with similar results.
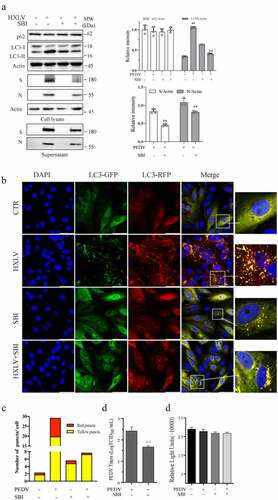
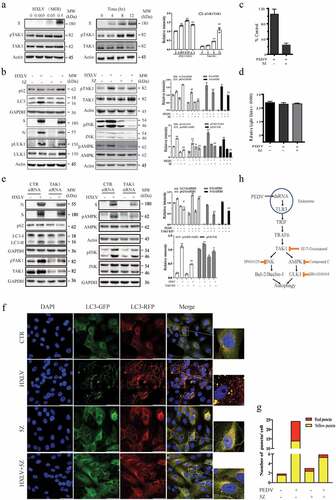
Figure 7. TAK1 activation contributes to PEDV-induced formation of autolysosomes and virus replication. (a) Vero cells were infected with the indicated MOI (0, 005, 0.01, 0.5) of PEDV HXLV strain for 12 hr or with 0.5 MOI for the indicated length of time. TAK1S412 phosphorylation, total TAK1 protein, and β-actin were detected by Western blot. (b) Uninfected or PEDV-infected Vero cells were treated with DMSO (0.5%) or 5Z (2 µM) and incubated for 12 hr. TAK1S412, AMPK, and JNK phosphorylation, LC3 lipidation, p62, and viral protein levels were analysed by Western blot. Viruses released into the conditioned media were titrated by measuring the TCID50 values (c). (d) 5Z has no effect on Vero cell viability. Uninfected or PEDV-infected Vero cells were treated with DMSO (0.5%)or 5Z (2 µM). After incubation for 12 hr, cell viability was quantified by using a CellTiter-Glo kit. (e) TAK knockdown blocks PEDV-induced autophagy. Vero cells were transfected with control or TAK1 siRNA were then incubated for 36 hr. After infection with HXLV virus (0.5 MOI) for 12 hr. LC3, p62, viral N and S proteins, TAK1S412 and other phosphorylated proteins were detected with the indicated antibodies. *p < 0.05, **p < 0.01, compared to uninfected control; #p <0.05,##p <0.01, compared to PEDV virus-infected cells. (f & g) GFP-RFP-LC3-transfected Vero cells were mock-infected or infected with PEDV (0.5 MOI). After incubation for 8 hr, the cells were treated with DMSO (0.5%) or 5Z (2 µM) d and incubated for another 8 hr. After nuclei staining with DAPI, orange autophagosomal puncta and red autolysosomal puncta were examined under a confocal microscope and quantified (f). The mean numbers of autolysosomes shown in red bars and autophagosomes shown in orange bars per cell were plotted in a bar graph (g). (h) the schematic model of PEDV-induced autophagy. PEDV viral RNA binds to endosomal TLR3, which then engages with TRIF and activates TRAF6 and its downstream kinase TAK1. TAK1 activates AMPK and JNK activation. AMPK phosphorylates ULK1 at S777 and activates it. JNK phosphorylates Bcl-2, leading to its dissociation with Beclin-1. Free Beclin-1 is then assembled into the pre-initiation complex. ULK1 phosphorylates VPS34, a class III PI-3 kinase in the pre-initiation complex, to induce autophagy. In addition to the TAK1-AMPK/JNK axis, accumulating evidence suggests that other signalling pathways such as ER stress and the PI-3 kinase pathway may participate in mediating PEDV-induced autophagy.
Supplemental Material
Download TIFF Image (1.3 MB)Data availability statement
The authors confirm that the data reported in this study are available within the article and/or its supplementary materials.
