Figures & data
Figure 1. Increased intracellular bacteriolysis of oatA-deficient Staphylococcus triggers more cell death. (a) Lactate dehydrogenase (LDH) release assay of mouse bone marrow derived macrophages (BMDM) infected with wild-type S. aureus USA300 (WT) at MOI = 50 for 6 hr, 24 hr, or 48 hr, compared to BMDMs without infection (NT). (b) Intracellular bacteria survival in BMDM infected with WT. T-test, ***p < 0.001. (c) Lysozyme sensitivity of WT, oatA deficient mutant (ΔoatA) and complemented mutant (δ:oata) by agar diffusion assay. (d) Intracellular bacteria survival in BMDM infected with WT or δoata. (e) LDH release assay of BMDM infected with WT, δoata or δ::oata at MOI = 50 for 6 hr, compared to NT. (f) Propidium iodide (PI) incorporation assay of BMDM infected with WT, δoata or δ::oata at MOI = 50 for 6 hr, compared to NT. (g) LDH release assay of peritoneal macrophage (PMΦ) infected with WT, δoata or δ::oata at MOI = 50 for 6 hr, compared to NT. (h) LDH release assay of PMΦ pretreated with lysozyme inhibitors (Leupeptin, or Leupeptin plus NH4Cl) and subsequently infected with δoata. All the experiments were repeated at least three times with at least two technical replicates for each experiment. one-way ANOVA, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
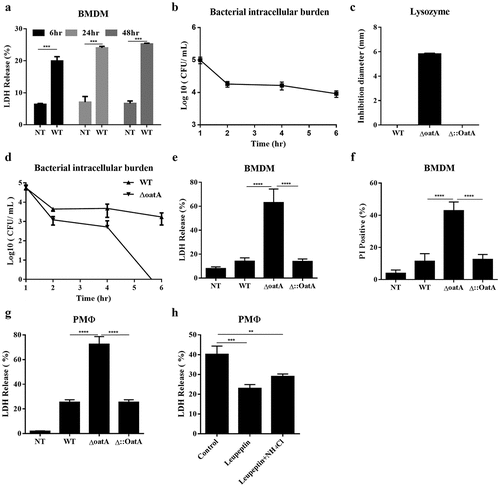
Figure 2. Intracellular bacteriolysis exacerbates AIM2-mediated programmed necrosis. (a) Western blot for cleaved-caspase-3 in BMDM infected with WT, δoata or δ:oata at MOI = 50 for 6 h, in contrast with BMDMs without infection (NT), + is the positive control. (b) Western blot for PARP-1 cleavage in BMDM infected with WT or δoata at MOI = 50 for 6 hr. Experiments were repeated three times. (c) Changes in the BMDM transcriptome infected with δoata or WT for 6 hr. Heatmap showed Log2 fold-changes of cell death and/or inflammatory response genes with a absolute Log2 (fold-change between δoata and WT stimulation group) > 1. (d) Western blot for AIM2 in BMDM infected with WT or δoata for 6 hr and 24 hr. LDH release (e) or PI incorporation (f) assays of C57 or AIM2-/- BMDM infected with WT, δoata or δ::oata at MOI = 50 for 6 hr. (g) Western blot for PARP-1 cleavage in C57 or AIM2-/- BMDM infected with WT or δoata at MOI = 50 for 6 hr and 24 hr. Each western blots was representative of several blots. The β-tubulin of (d) and (g) are identical because the representatives are from the same sample. Each cell death assay in each condition was repeated at least three times with at least two technical replicates. Two-way ANOVA, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
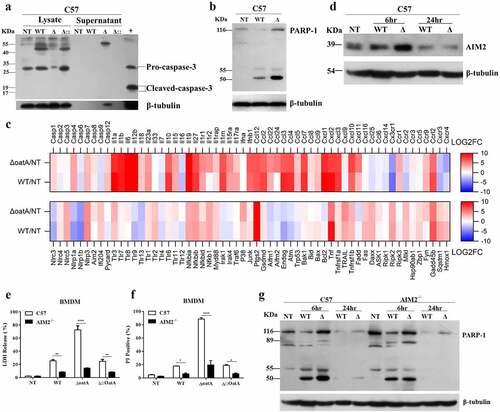
Figure 3. Intracellular bacteriolysis exacerbates AIM2 inflammasome activation, but not pyroptosis. (a) Western blot for cleaved-caspase-1 in lysate and supernatant of C57 BMDM infected with WT, δoata or δ:oata at MOI = 50 for 6 hr. (b) Western blot for cleaved-caspase-1 in supernatant of C57 or AIM2-/- BMDM infected with WT, δoata or δ::oata at MOI = 50 for 6 hr. (c) Western blot for cleaved-caspase-1 in supernatant of C57 or NLRP3-/- BMDM infected with WT, δoata or δ::oata at MOI = 50 for 6 hr. ELISA assay for IL-1β release of C57, AIM2−/− or NLRP3−/− BMDM pretreated with LPS for 4 hr and subsequently infected with WT or δoata at MOI = 50 for 2 hr (d) or 6 hr (e). (f) C57 or AIM2−/− mice (n = 4) were injected subcutaneously in opposite flanks of notum with 3 × 106 CFU of WT or δoata, and skin lesion size was measured at the indicated time. (g) a representative pair of lesions caused by WT or δoata after 2 days is depicted. (h) LDH release assay of C57 or NLRP3−/− BMDM infected with WT, δoata or Δ::OatA at MOI = 50 for 6 h. (i) LDH release assay of C57, caspase-1/11−/− or GSDMD−/− BMDM infected with WT or δoata at MOI 50 for 6 hr. (j) LDH release assay of C57 or GSDMD−/− PMΦ infected with WT, δoata or Δ::OatA at MOI 50 for 6 hr. Western blots were representative of several blots. ELISA assay was repeated twice with three technical replicates. Cell death assay was repeated three times with three technical replicates. Two-way ANOVA, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
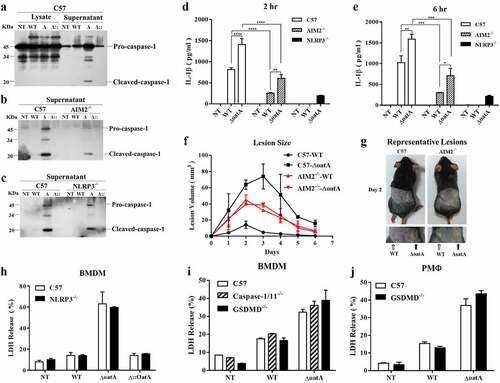
Figure 4. Intracellular bacteriolysis exacerbates AIM2-mediated necroptosis. (a) Western blot for RIPK3 and pMLKL in C57 BMDMs infected with WT or δoata at MOI 50 for 6 hr. (b) Western blot for RIPK3 in C57 BMDM pretreated with 50 μM nec-1 or DMSO for 1 hr and subsequently infected with WT or δoata at MOI 50 for 6 hr. (c) PI incorporation assays of C57 BMDM pretreated with 50 μM Nec-1, 20 μM VX-765 or DMSO and subsequently infected with WT or δoata at MOI 50 for 6 hr. (d) LDH assay of C57 or MLKL−/− BMDM infected with WT or δoata at MOI 50 for 6 hr. (e) Western blot for cleaved-caspase-1 in C57 or MLKL−/− BMDM infected with WT, δoata or δ:oata at MOI 50 for 6 hr. (f) Western blot for pMLKL in C57 or caspase-1/11−/− BMDM infected with WT, δoata or δ::oata at MOI 50 for 6 hr. (g) Gray scanning analysis of Fig. 4 G. (h) Western blot for RIPK3 and pMLKL in C57 or AIM2-/- BMDM infected with WT or δoata at MOI 50 for 6 hr or 24 hr. (i) & (j) Gray scanning analysis of Fig. 4 H. Western blots were representative of several blots. Cell death assays were repeated three times with three technical replicates. Two-way ANOVA, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
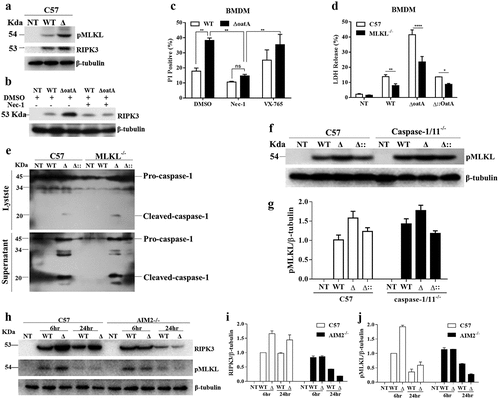
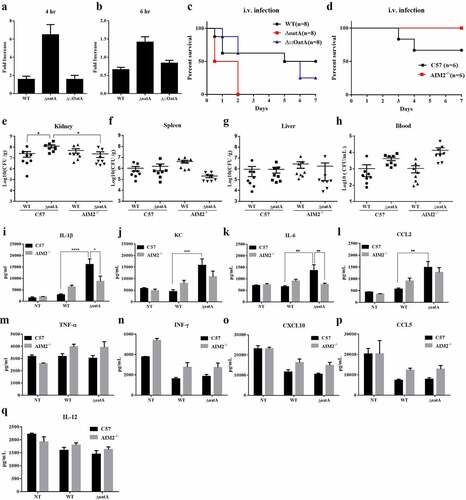
Figure 5. Increased intracellular bacteriolysis contributes to S. aureus pathogenicity. Extracellular bacterial change of BMDM infected with WT, ΔoatA or Δ::OatA at MOI=50 for 4 hr (a) or 6 hr (b) , compared with control group that had no cells and added bacteria. The experiments were repeated twice with three technical replicates. (c) Survival in mice following the tail intravenous (i.v.) injection of 1×108 colony-forming units (CFU) of WT, ΔoatA or Δ::OatA. (d) Survival in C57 and AIM2-/- mice following i.v. injection of 0.2×108 CFU of ΔoatA. Bacterial burden in kidney (e), spleen (f), liver (g) and blood (h) of C57 or AIM2-/- mice following i.v. of 0.5×108 CFU of WT or ΔoatA after 48 hr (n=8 per group, one-way ANOVA, *P<0.05). Pro-inflammatory mediators in kidney of C57 or AIM2-/- mice at 48 hr post i.v. infection of 0.5×108 CFU of WT or ΔoatA were examined by ELISA assay : (i) IL-1β, (j) KC, (k) IL-6, (l) CCL2, (m) TNF-α, (n) IFN-γ, (o) CXCL10, (p) CCL5, (q) IL-12. Two-way ANOVA, *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
Supplemental Material
Download Zip (431.6 KB)Data availability statement
Data sharing is not applicable to this article as no new data were created or analysed in this study
