Figures & data
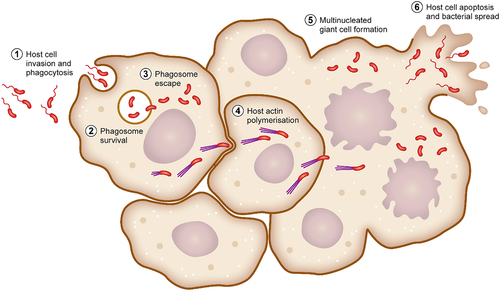
Figure 1. Determinants required for B. pseudomallei attachment, invasion and phagosomal survival. B. pseudomallei uses a variety of mechanisms to initiate infection of host cells. Organelles and proteins such as the flagella, fimbriae, TFP and TAAs are used to attach to and directly infect host cells. In addition, B. pseudomallei is phagocytosed by macrophages. Following phagocytosis, B. pseudomallei induces expression of a plethora of proteins involved in phagosomal survival which as OxyR, KatG and AphC which protects against reactive oxygen species. Phagosomal escape is subsequently mediated by the type III secretion system which allows for rapid escape into the cytoplasm.
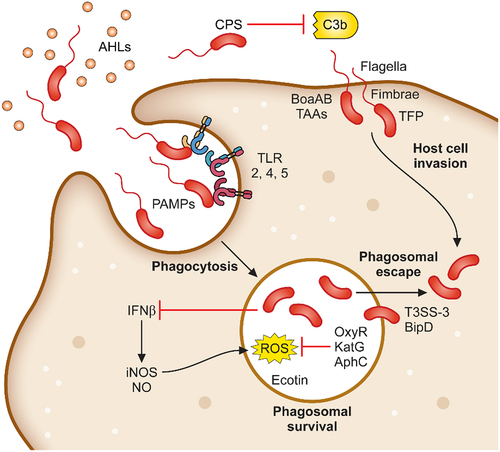
Table 1. Polysaccharides produced by B. pseudomallei.
Figure 2. The intracellular adaptation and spread of B. pseudomallei from cell-to-cell. Following phagosomal escape B. pseudomallei switches its metabolic pathways to allow for the greatest energy production within the cytoplasmic environment. Additionally, secretion of the toxin BLF1 and the T3SS effector CHBP modulate host cell processes, halting host protein synthesis. Host cell actin is polymerised by BimAC and B. pseudomallei cells rapidly propel to the cellular membrane upon which their T6SS–1 is expressed and Hcp1 causes membrane fusion and the formation of multinucleated giant cells (MNGCs). MNGC formation facilitates rapid spread of bacterial infection to neighbouring cells while escaping recognition by the immune response.
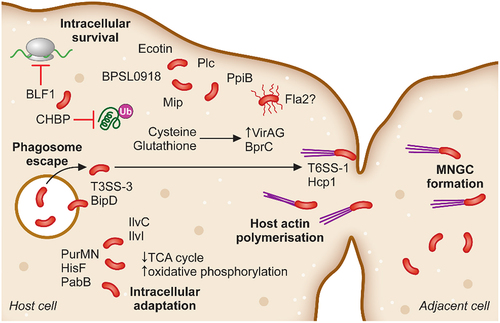
Table 2. Type VI secretion systems (T6SS) of B. pseudomallei. B. pseudomallei encodes for six T6SS clusters as determined by homology to cluster of orthologous genes (COG) in other Gram-negative pathogens. Two nomenclature systems for B. pseudomallei T6SS were published in 2007, Schell et al. and Shalom et al. [Citation190,Citation191]. Both nomenclatures are listed as well as the known functions of these clusters in B. pseudomallei.
