Figures & data
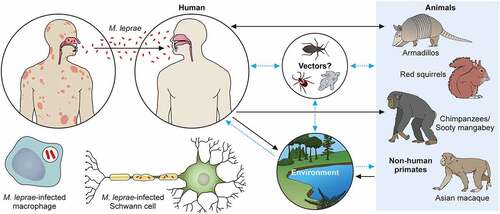
Figure 1. The structure of the M. leprae cell wall. The M. leprae cell wall consists of an inner and outer layer that surround a plasma membrane. The outermost layer includes PGLs that compose capsules. The electron-dense inner layer of cell wall contains PGN, AG, and mycolic acids. The outer cell wall, which is an electron-dense layer, consists of lipid-linked polysaccharides such as LAM, LM, and phthiocerol-containing lipids including phthiocerol dimycocerosate and dimycolyl trehalose. Mycolic acids link to arabinan chain termini and compose the inner leaflet of a pseudo lipid bilayer. An outer leaflet contains TMM mycolic acids and PDIM and PGL mycocerosoic acids. Small amounts of TMM also exist in the cell wall of M. leprae.
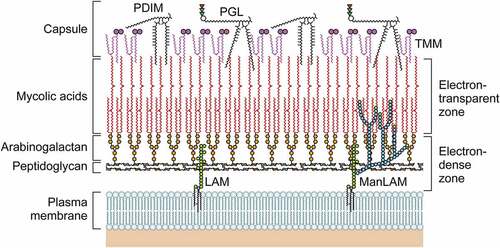
Figure 2. Classification of leprosy on the Ridley-Jopling scale based on immunology, histology, and bacteriology. Leprosy is classified into tuberculoid (TT), borderline tuberculoid (BT), mid-borderline (BB), borderline lepromatous (BL), and lepromatous leprosy (LL). TT leprosy showing strong cell mediated immunity is characterized by granulomatous skin lesions infiltrated predominantly by lymphocytes and epithelioid cells along with high secretion of the Th1 cytokines IL2, and IFN-γ in TT/BT lesions. Conversely, in BL/LL a high level of humoral immunity with a low level of cell mediated immunity is exhibited predominantly by macrophage granulomas with foamy macrophages with few lymphocytes, high levels of Treg cells along with numerous acid-fast bacilli and secretion of high levels of IL-4, IL-5 and IL-10.
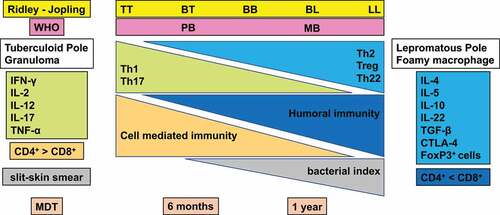
Figure 3. The transmission pathways of M. leprae. The M. leprae transmission pathways are not fully clear. However, an increased risk of human-to-human transmission because of intimate communication between untreated leprosy patients has been noted. Spreading via infectious aerosols is considered to be the most likely route of infection. M. leprae invades skin macrophages and Schwann cells, inducing skin lesions and neurological injury. Zoonotic transmission of M. leprae due to natural infection of armadillos in the Southeast United States has been reported, and humans and armadillos share a specific M. leprae strain. Red squirrels (Sciurus vulgaris) in the British Isles harbour M. leprae. Non-human primates including chimpanzees (Pan troglodytes) have been detected with leprosy in Africa and Asia. It has been speculated that potential vectors, such as amoebae, kissing bugs, and ticks, as well as the environment, could be potential transmission routes for M. leprae as a zoonotic disease. Black dotted arrows show confirmed transmission pathways. Grey arrows show hypothetical transmission pathways. Red dotted arrows show the main route of transmission between humans. An aerosol spreads the nasal secretions.
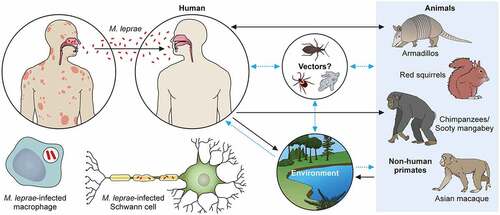
Figure 4. Histopathology of leprosy. (a) TT is characterized by granulomas with lymphocyte infiltration. These are multiple, well-formed granulomas with multinuclear Langhans giant cells. Erosion of the basal layer of epidermis is observed, with lymphocytes (→). (HE stain, 40×). (b) in BT lesions a granulomatous appearance can be observed (→), similar to TT lesions, with the presence of a grenz zone. Lymphocytic infiltration is less than in TT. (HE stain, 40×). (c) in BL cases, lymphocytic infiltration and histiocytes (→) with granular to foamy cytoplasm are observed. (HE stain, 40×). (d) LL is characterized by foamy histiocytes with a grenz zone below the epidermis. (→) (HE stain, 40×) (A) the slit skin smear test shows the acid fast bacilli. (→) (Ziehl- Neelsen stain, 1000×). (B) a large number of bacilli are observed within foamy histiocytes with LL lesions. (→) (Ziehl- Neelsen stain, Wade-Fite, 400×). Photomicrographs are courtesy of Dr. Norihisa Ishii, National Sanatorium Tama Zenshoen.
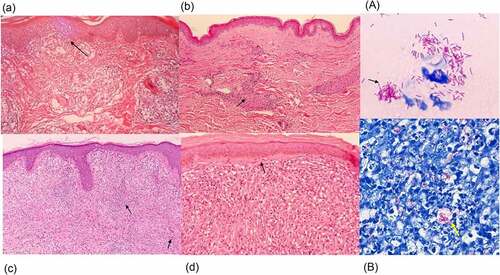
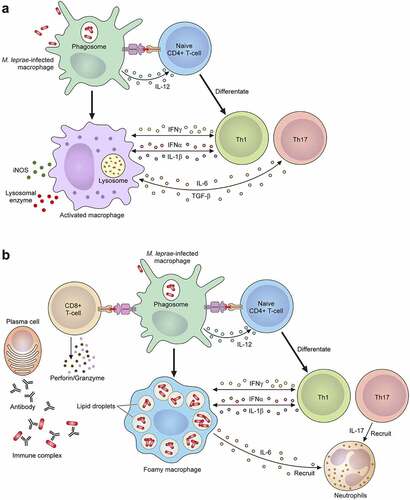
Figure 5. The mechanism of the leprosy reaction. (a) T1 R is led by a cellular immune response mediated via CD4+ T cells. Activated macrophages release pro-inflammatory Th1 cytokines, such as IFN-γ, TNF-α, IL-1β, IL-6, IL-2, IL-12, TGF-β and iNOS, which cause tissue damage. (b) ENL is a generalized proinflammatory reaction featuring the infiltration of neutrophils. The activation of complement, immune complexes, increasing CD4+/CD8+ T cell subset ratios and high degree of proinflammatory cytokines including TNF-α in the lesions and in the circulation can also be observed. ENL shows low cellular immunity, but there are enough B cells and plasma cells to produce antibodies against M. leprae. In the acute stage of ENL lesions, a large number of neutrophilic infiltrations is observed. An activated CD8+ T cell secretes cytotoxic granule proteins such as perforin and granzymes which lead to apoptosis of the cells.