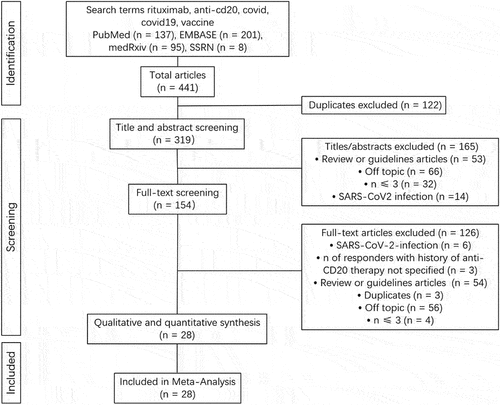
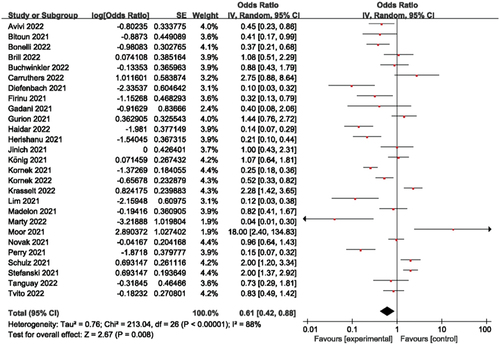
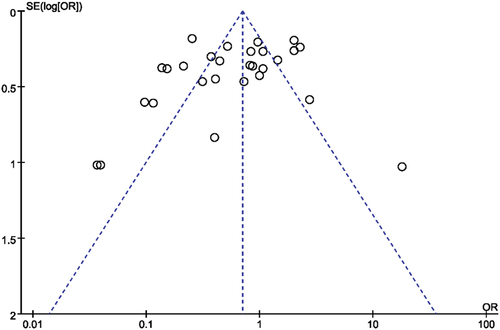
Figures & data
Table 1. List of the included studies.
Figure 3. Humoral immune responses according to prespecified subgroups of 3-6, 6, 6–9 and 9–12 months since the last dose of anti- CD20 therapy.
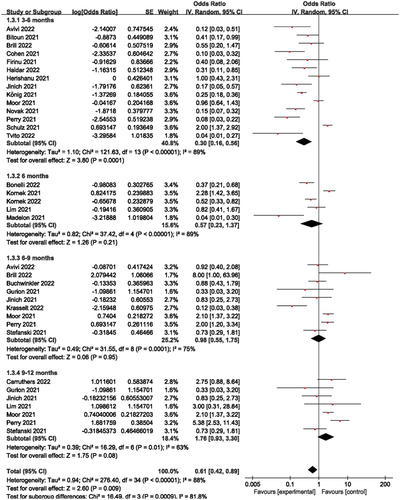
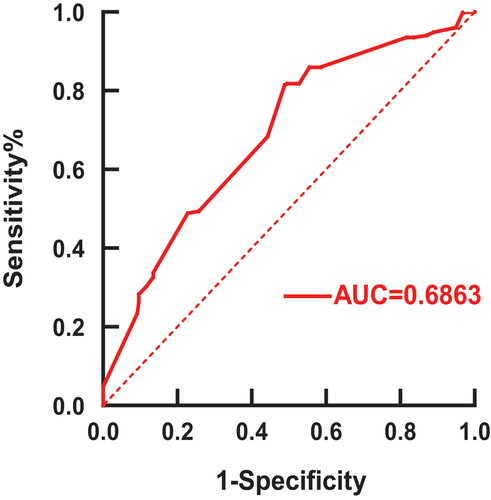
Figure 4. ROC curve on the time interval of SARS-CoV-2 vaccination in 1455 patients treated with anti-CD20.The vertical coordinates indicate that all patients who actually received the vaccine were greater than 5.5 months from the last dose of anti-CD20 monoclonal antibody therapy were not infected with the COVID-19. The abscissa indicates that all patients who actually received the vaccine were more than 5.5 months from the last dose of anti-CD20 monoclonal antibody therapy were infected with the COVID-19.