Figures & data
Figure 1. Overview of virulence in African trypanosomes. (from left to right); Human infectivity: ApoL1 is the main component of human trypanosome lytic factor (TLF), a high-density lipoprotein subclass that confers protection against animal-infective trypanosomes through parasite lysis. The human-infective trypanosome species, T. b. rhodesiense and T. b. gambiense, have evolved mechanisms to evade ApoL1-mediated lysis, strongly influencing virulence in human hosts. For example, T. b. rhodesiense can express SRA, a protein that neutralises ApoL1 through direct interaction. Another mechanism is reduced ApoL1 uptake via an L210S mutation in the haptoglobin-haemoglobin receptor (HpHbr) that inactivates it. Coinfection: Infection with multiple species and/or strains can lead to multiple virulence phenotypes as described. For example, the presence of a less virulent strain can suppress the pathology associated with a more virulent strain of the same species in a coinfection setting. In addition, coinfection of multiple trypanosome species can impact differentiation dynamics. Immune response: The interaction of trypanosomes and the host immune response can greatly impact virulence phenotypes. Antigenic variation is undoubtedly a paradigm of trypanosome biology. Hydrodynamic flow of VSGs across the cell surface sweep bound antibodies to the cell posterior, where they are degraded following endocytosis. Furthermore, trypanosomes regularly switch the identity of the expressed VSG, leading to waves of parasitaemia with host antibodies eventually raised to the dominant VSG in the parasite population. A further parasite virulence phenotype associated with the host immune response is the ablation of B cell memory via killing of host B cells. Extravasation/sequestration: A key symptom of HAT is an ability of T. brucei to extravasate and enter extravascular tissues, in particular the brain, adipose tissue and the skin. A related virulence phenotype has also been described in animal-infective trypanosomes, albeit caused by intravascular sequestration rather than extravasation (e.g. strain and tissue specific sequestration in T. congolense). Secreted factors/EVs: Trypanosomes release a significant amount of metabolites, proteins and vesicles into the host environment, several of which have been characterised. In particular, virulence associated with secreted peptidases has been established, with oligopeptidase B (targeting atrial natriuretic factor), type 1 proglutamyl peptidase (targeting gonadotropin-releasing hormone and thyrotropin-releasing hormone) and prolyl oligpeptidase (type I and native collagen) all targeting host effectors. As-of-yet unidentified parasite-derived secretome components also target the maturation of host LPS-induced dendritic cells. Abbreviations: ApoL1: apolipoprotein L1; HpHbr: haptoglobin-haemoglobin receptor; SRA: serum resistance-associated protein; VSG: variant surface glycoprotein; OPB: oligopeptidase B; ANF: atrial natriuretic factor; PGP: proglutamyl peptidase; GnRH: gonadotropin-releasing hormone; TRH: thyrotropin-releasing hormone; POP: prolyl oligpeptidase; COL I: type I collagen; COL-N: native collagen; LPS: lipopolysaccharide; EVs: extracellular vesicles. Inset graph in immune response panel adapted from [Citation6].
![Figure 1. Overview of virulence in African trypanosomes. (from left to right); Human infectivity: ApoL1 is the main component of human trypanosome lytic factor (TLF), a high-density lipoprotein subclass that confers protection against animal-infective trypanosomes through parasite lysis. The human-infective trypanosome species, T. b. rhodesiense and T. b. gambiense, have evolved mechanisms to evade ApoL1-mediated lysis, strongly influencing virulence in human hosts. For example, T. b. rhodesiense can express SRA, a protein that neutralises ApoL1 through direct interaction. Another mechanism is reduced ApoL1 uptake via an L210S mutation in the haptoglobin-haemoglobin receptor (HpHbr) that inactivates it. Coinfection: Infection with multiple species and/or strains can lead to multiple virulence phenotypes as described. For example, the presence of a less virulent strain can suppress the pathology associated with a more virulent strain of the same species in a coinfection setting. In addition, coinfection of multiple trypanosome species can impact differentiation dynamics. Immune response: The interaction of trypanosomes and the host immune response can greatly impact virulence phenotypes. Antigenic variation is undoubtedly a paradigm of trypanosome biology. Hydrodynamic flow of VSGs across the cell surface sweep bound antibodies to the cell posterior, where they are degraded following endocytosis. Furthermore, trypanosomes regularly switch the identity of the expressed VSG, leading to waves of parasitaemia with host antibodies eventually raised to the dominant VSG in the parasite population. A further parasite virulence phenotype associated with the host immune response is the ablation of B cell memory via killing of host B cells. Extravasation/sequestration: A key symptom of HAT is an ability of T. brucei to extravasate and enter extravascular tissues, in particular the brain, adipose tissue and the skin. A related virulence phenotype has also been described in animal-infective trypanosomes, albeit caused by intravascular sequestration rather than extravasation (e.g. strain and tissue specific sequestration in T. congolense). Secreted factors/EVs: Trypanosomes release a significant amount of metabolites, proteins and vesicles into the host environment, several of which have been characterised. In particular, virulence associated with secreted peptidases has been established, with oligopeptidase B (targeting atrial natriuretic factor), type 1 proglutamyl peptidase (targeting gonadotropin-releasing hormone and thyrotropin-releasing hormone) and prolyl oligpeptidase (type I and native collagen) all targeting host effectors. As-of-yet unidentified parasite-derived secretome components also target the maturation of host LPS-induced dendritic cells. Abbreviations: ApoL1: apolipoprotein L1; HpHbr: haptoglobin-haemoglobin receptor; SRA: serum resistance-associated protein; VSG: variant surface glycoprotein; OPB: oligopeptidase B; ANF: atrial natriuretic factor; PGP: proglutamyl peptidase; GnRH: gonadotropin-releasing hormone; TRH: thyrotropin-releasing hormone; POP: prolyl oligpeptidase; COL I: type I collagen; COL-N: native collagen; LPS: lipopolysaccharide; EVs: extracellular vesicles. Inset graph in immune response panel adapted from [Citation6].](/cms/asset/99c711e6-b6db-455b-970f-0d430bd2467f/kvir_a_2150445_f0001_oc.jpg)
Figure 2. Parasite metabolism and virulence. A) Trans-sialidases released by T. vivax cleave sialic acid moieties from glycoproteins on the erythrocyte cell surface, leading to erythrophagocytosis and eventually, anaemia. B) All three species of pathogenic African trypanosomes are known to release phospholipases that degrade phosphocholine-bound lipids. They are considered significant virulence factors, and their action results in a build up of choline in the host bloodstream. C) T. brucei secretes multiple factors that modulate macrophage ability to generate nitric oxide (NO), including TbKHC1, and soluble VSG (sVSG). The latter stimulates arginase-1 activity, leading to increased usage of the available arginine pool to generate ornithine, reducing substrate availability for NO production through nitric oxide synthase (NOS). Simultaneously, sVSG has an inhibitory effect on NOS. sVSG also interferes with the phosphorylation of STAT1, an important transcription factor that drives pro-inflammatory responses. D) Parasite amino acid metabolism and its effect on host responses has been studied to some degree in T. brucei. In particular the fate of hydroxyphenylpyruvate (HPP), phenylpyruvate (PP) and indolepyruvate (IP), products of cASAT-catalysed conversions of L-tyrosine, L-phenylalanine and L-tryptophan, respectively. IP is a potent modulator of pro-inflammatory responses in macrophages. Firstly, IP interferes with HIF-1α, leading to a reduction in glycolytic capacity of macrophages. Secondly, IP inhibits induction of pro-IL-1, a potent pro-inflammatory cytokine. Finally, more recent work has established that IP is a direct inhibitor of cyclooxygenase (COX), leading to reduced prostaglandin (PG; mediators of inflammation) production. E) Trypanosome-derived IP as well as HPP can impact upon dendritic cells, by stimulating Nrf2-mediated hemeoxygenase-1 (HO-1) induction, again leading to a reduced pro-inflammatory response. Many other metabolic factors are known to be excreted by trypanosomes, but their molecular interactions with the host environment remain to be established, and they are therefore not included in this overview figure.
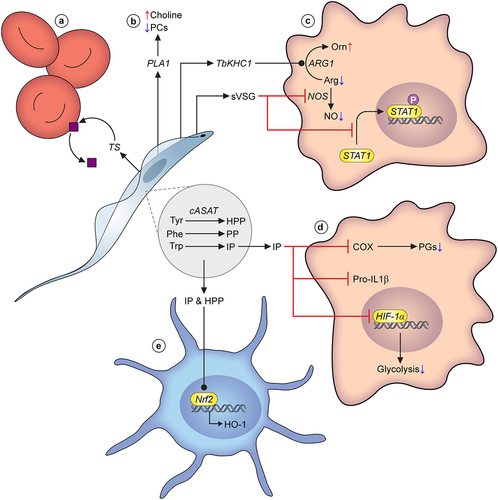
Figure 3. Quorum sensing in Trypanosoma brucei. Schematic pathway for the quorum sensing signalling pathway in Trypanosoma brucei. Slender form parasites release several peptidases into their environment, with two peptidases, Oligopeptidase B and Metallocarboxypeptidase I being important contributors to the generation of the quorum sensing signal, oligopeptides. Environmental oligopeptides can be transported into recipient parasites by the TbGPR89 surface transporter that is expressed on slender cells but not stumpy forms. In an unknown mechanism, transported oligopeptides stimulate a signal transduction cascade that promotes stumpy formation through the action of gene regulators (RNA binding proteins). Molecules that act to inhibit stumpy formation (slender retainers) are inactivated. At least one kinase, TbDYRK, acts on both control arms, inhibiting slender retainers and promoting stumpy formation. Other molecules, annotated as “Hypothetical proteins” in TryTrypdb (https://tritrypdb.Org/tritrypdb/app) have been identified that control stumpy formation but their positions in the regulatory pathway are unknown.
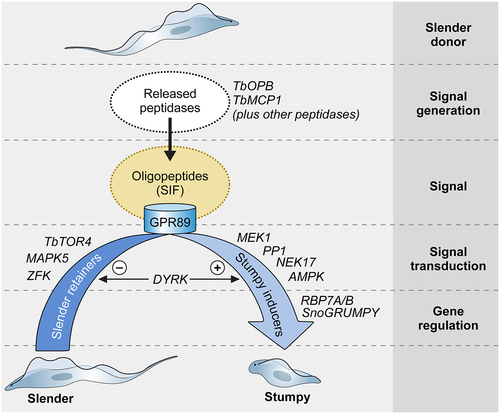
Table 1. Virulence phenotypes and state of current knowledge.
