Figures & data
Figure 1. Global distribution of mpox at the human-animal interface. The upper global map shows the outbreak distribution countries before 2022 and the lower global map shows the outbreak distribution countries in 2022.
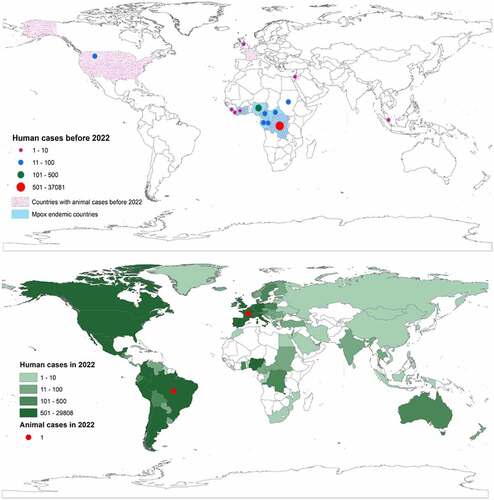
Table 1. Reported non-human primate, rodent, and other species positive with mpox either by antibody to the virus, nucleotide detection, or virus isolation before 2022.
Figure 2. Origin and transmission dynamics of mpox. The origin of the virus or its natural reservoir is unknown. Rodents and non-human primates are susceptible to the virus, and humans can get the infection if get contact with infected humans, animals, or the environment when travelling to endemic countries, handling infected animals during bushmeat consumption or business, or for other purposes. Human-to-human transmission is possible in close contact, nosocomial, or family settings. There are recent reports of human-to-dog transmission.
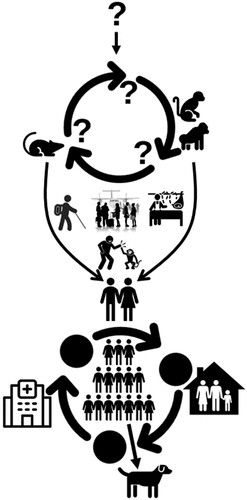
Figure 3. Cell-level replication cycle of mpox virus and mechanism of action of antivirals. The replication takes place in the cytoplasm of the host cell called the viral factory, following entry of a mature virion by micropinocytosis/fusion and enveloped virion by fusion method. After entry, the virion gets uncoated and the viral genome, protein, and enzyme are released to the host cytoplasm. The protein and enzyme initiate the replication process and prevent the cell defense to prevent replication. After a series of early, intermediate, and late phases of genomic replication, viral elements are assembled to form an immature virion, which later converts to a mature virion. The mature virion then gets a secondary membrane from the trans-Golgi network to form enveloped virion. The antiviral drugs “Cidofovir” and “Brincidofovir” prevents viral replication, whereas “Tecovirimat” prevents the envelope wrapping in Golgi.
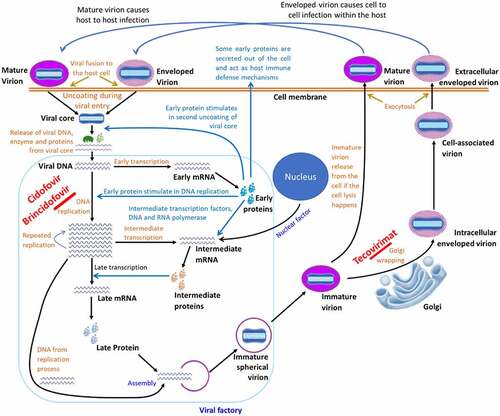
Figure 4. Pathogenesis of mpox virus in humans. After entry into the body, the virus replicates at the inoculation site and in the local lymph nodes. Primary viremia leads the viral spread towards other organs, like the liver and spleen, and replicates. Early clinical features start at this stage. When the secondary viremia takes place and the virus is transferred towards the cutaneous parts of the body, then mucosal and other lesions develop [Citation98]. The molecular diagnosis is possible from the stage of primary viremia until the pustule formation. The antibody IgM can be detected when the secondary viremia starts till the lesions are resolved, whereas the IgG can be detected from the third week of infection till a year depending on the patient’s immune status. Viral shedding can happen with the beginning of clinical symptoms; however, the risk period of viral shedding is the vesicular and pustular stages.
![Figure 4. Pathogenesis of mpox virus in humans. After entry into the body, the virus replicates at the inoculation site and in the local lymph nodes. Primary viremia leads the viral spread towards other organs, like the liver and spleen, and replicates. Early clinical features start at this stage. When the secondary viremia takes place and the virus is transferred towards the cutaneous parts of the body, then mucosal and other lesions develop [Citation98]. The molecular diagnosis is possible from the stage of primary viremia until the pustule formation. The antibody IgM can be detected when the secondary viremia starts till the lesions are resolved, whereas the IgG can be detected from the third week of infection till a year depending on the patient’s immune status. Viral shedding can happen with the beginning of clinical symptoms; however, the risk period of viral shedding is the vesicular and pustular stages.](/cms/asset/14b5b31c-5ee6-4820-9c72-43d595e72619/kvir_a_2186357_f0004_oc.jpg)