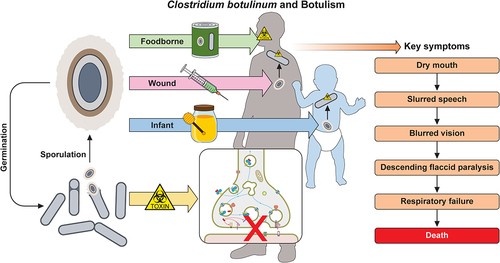
Figures & data
Figure 1. Classification of C. botulinum and the other BoNT producing strains. (a) A phylogenetic dendrogram of a representative selection of strains producing the seven different serotypes of BoNT and other related clostridia (NT – nontoxigenic strain). Constructed by average nucleotide identify from published whole genome assembly sequences and neighbour-joining tree method (b) Dendrogram comparing the protein sequences of all BoNT toxin types including the tetanus toxin (TeNT). Amino Acid sequences analysed by ClustalW alignment and tree created using the Maximum Likelihood method. (c) Table summarising the Clostridium spp. known to produce the BoNT; depicting the six phylogenetically distinct BoNT neurotoxic clostridia. Colours throughout refer to the group the strain or toxin type belongs to. All evolutionary analyses were conducted in MEGA11, with prior multiple sequence alignment of whole genomes by CLC Genomics Workbench 21.0.3 (CLC Bio, Aarhus, Denmark).
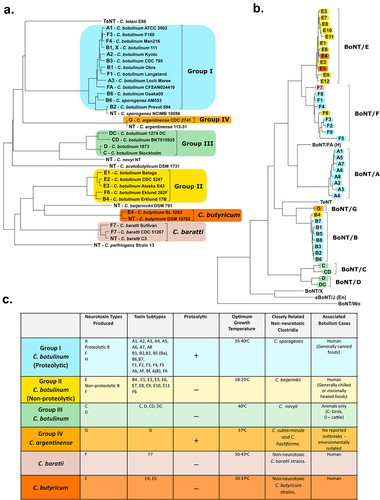
Figure 2. Reported botulism cases in the U.S. from 2001–2018. (a) Graph depicts the number of cases of each type of botulism, demonstrating the prevalence of infant botulism compared to other clinical types. (b) Shows the causative toxin type of reported cases if specified. All data obtained from the CDC national botulism surveillance annual summaries [Citation110].
![Figure 2. Reported botulism cases in the U.S. from 2001–2018. (a) Graph depicts the number of cases of each type of botulism, demonstrating the prevalence of infant botulism compared to other clinical types. (b) Shows the causative toxin type of reported cases if specified. All data obtained from the CDC national botulism surveillance annual summaries [Citation110].](/cms/asset/7cfe6647-edbb-4693-900c-13da55a0a423/kvir_a_2205251_f0002_oc.jpg)
Figure 3. The classical structure of the BoNTs. Depicted is the BoNT/A1 from c. botulinum ATCC 3502. The bontA1 gene is translated into a single 1295 amino acid polypeptide (c.), which is cleaved into a light chain (L – coloured red) and a heavy chain joined by a disulphide bridge between Cys430 and Cys454 residues (shown in black). The heavy chain contains an N-terminal translocation domain (HN – coloured light green) and a C-terminal receptor binding domain, which is formed of two subunits (HC-N and HC-C coloured dark and light blue respectively). The light chain is the catalytic domain and consists of a zinc metalloprotease (the Zn2+ atom shown in yellow). The light chain is encircled by a hydrophobic peptide belt domain of the HN (coloured in dark green in c.). Crystal structures were prepared using UCSF ChimeraX (PBD ID: 3BTA) with a. displayed in ribbon format and b. a spacefill representation [Citation198,Citation202].
![Figure 3. The classical structure of the BoNTs. Depicted is the BoNT/A1 from c. botulinum ATCC 3502. The bontA1 gene is translated into a single 1295 amino acid polypeptide (c.), which is cleaved into a light chain (L – coloured red) and a heavy chain joined by a disulphide bridge between Cys430 and Cys454 residues (shown in black). The heavy chain contains an N-terminal translocation domain (HN – coloured light green) and a C-terminal receptor binding domain, which is formed of two subunits (HC-N and HC-C coloured dark and light blue respectively). The light chain is the catalytic domain and consists of a zinc metalloprotease (the Zn2+ atom shown in yellow). The light chain is encircled by a hydrophobic peptide belt domain of the HN (coloured in dark green in c.). Crystal structures were prepared using UCSF ChimeraX (PBD ID: 3BTA) with a. displayed in ribbon format and b. a spacefill representation [Citation198,Citation202].](/cms/asset/bd97d302-939a-464a-9119-c442d032711a/kvir_a_2205251_f0003_oc.jpg)
Figure 4. Structure of the progenitor complex (PC) and BoNT-NTNH heterodimer. The NTNH and BoNT interlock to form a heterodimer with the catalytic light chain positioned on the exterior, held in place by the peptide belt of the heavy chain translocation domain (HN). The HA proteins assemble to form a symmetric tripod structure (B.). The NTNH interacts with the three HA70 proteins of each “arm” to complete the PC. Crystal structure of BoNT/A1- NTNH/A1 is shown in the bottom right and was prepared with UCSF ChimeraX with the BoNT heavy chain coloured dark red (PDB ID:3VOA) [Citation202,Citation216].
![Figure 4. Structure of the progenitor complex (PC) and BoNT-NTNH heterodimer. The NTNH and BoNT interlock to form a heterodimer with the catalytic light chain positioned on the exterior, held in place by the peptide belt of the heavy chain translocation domain (HN). The HA proteins assemble to form a symmetric tripod structure (B.). The NTNH interacts with the three HA70 proteins of each “arm” to complete the PC. Crystal structure of BoNT/A1- NTNH/A1 is shown in the bottom right and was prepared with UCSF ChimeraX with the BoNT heavy chain coloured dark red (PDB ID:3VOA) [Citation202,Citation216].](/cms/asset/821959ef-c5d6-4b3f-ac82-db1e5b1e8ab7/kvir_a_2205251_f0004_oc.jpg)
Figure 5. The mechanism of BoNTs at nerve endings. The signalling between the pre and postsynaptic cell is mediated by small molecules called neurotransmitters, at neuromuscular junctions (NMJ) this is acetylcholine. The neurotransmitter is stored in membrane bound synaptic vesicles inside the neuronal cytosol. Endocytosis of the empty vesicles triggers a V-ATPase proton pump to generate a pH gradient across the vesicular membrane, which drives the newly synthesised neurotransmitter molecules to enter the vesicles. The loaded vesicles bind to the interleaflet of the presynaptic membrane by the VAMP and synaptotagmin proteins in a process called docking. On docking, SNARE complexes form around the vesicle, and facilitate the fusing of the vesicle and the membrane in mechanism known as priming. As a result of a Ca2+ influx, caused by the depolarisation of the presynaptic nerve, SNARE proteins undergo a conformational change to allow the primed vesicle to release its neurotransmitter into the synaptic cleft. Through diffusion the neurotransmitter binds to receptors on the postsynaptic cell membrane, causing its excitation. This cycle is then repeated in readiness for future depolarisations. However, BoNT blocks neurotransmitter release from the presynaptic nerve terminal. The toxin is endocytosed along with empty vesicles and the light chain is translocated into the neuronal cytosol. This catalytic protein then cleavages SNARE proteins on the vesicular membrane or inner leaflet of the presynaptic membrane, dependent on the toxin serotype. This prevents vesicle docking and fusion to the presynaptic membrane and subsequently the release of neurotransmitter.
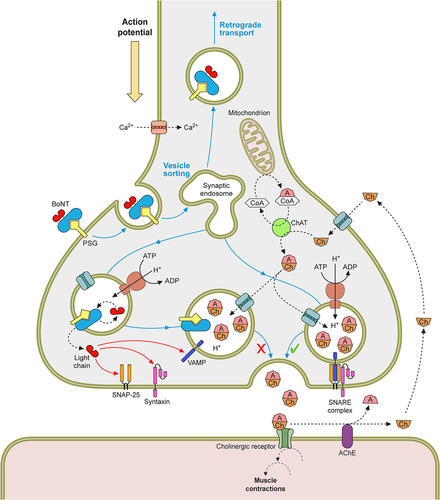
Figure 6. Alternative SNARE cleavage sites of BoNT serotypes. Specific residues cleaved in each of the SNARE targets and their respective BoNT serotypes are shown in the table.
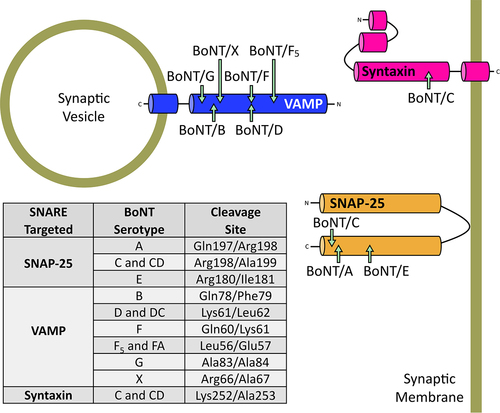
Figure 7. Botulinum neurotoxin gene cluster variation in a representative selection of neurotoxic strains. the diversity in the gene clusters and flanking regions, with ha clusters at the top and orfX at the bottom. The location of the gene cluster is indicated, if not chromosomal, on the left. The Group I strains either have the classical ha70-ha17-ha33-botR-ntnh-bont genes or the orfX3-orfX2-orfX1-botR-p47-ntnh-bont cluster. Group III are contained in ha clusters, although the alternative sigma factor gene, botR, is upstream of ha70. Group IV C. argentinese strains also have ha accessory proteins but the ha70 and ha33 genes are inverted relative to the conventional structure. Group II strains (with the exception of bont/B4 containing a ha70-ha17-ha33-botR-ntnh-bont cluster) have orfX3-orfX2-orfX1-p47-ntnh-bont clusters, which lack the botR gene completely; which is also observed in C. baratii F7 and the neurotoxic C. butyricum strains [Citation267,Citation268]. This diversity is likely attributable to recombination events and horizontal gene transfer between strains, which have also led to neurotoxin gene clusters present in non-Clostridium sp. such as Enterococcus sp. 3G1_DIV0629 and Weissella oryzae SG25 [Citation59]. The Weissella neurotoxin gene is present without accessory genes, the ntnh-like gene downstream of bont/Wo lacks significant domains compared to the well conserved ntnh [Citation269]. The insertion elements (IS) and flagellin genes flanking and internal to the gene clusters indicate the probable method of transfer into the strain as it evolved.
![Figure 7. Botulinum neurotoxin gene cluster variation in a representative selection of neurotoxic strains. the diversity in the gene clusters and flanking regions, with ha clusters at the top and orfX at the bottom. The location of the gene cluster is indicated, if not chromosomal, on the left. The Group I strains either have the classical ha70-ha17-ha33-botR-ntnh-bont genes or the orfX3-orfX2-orfX1-botR-p47-ntnh-bont cluster. Group III are contained in ha clusters, although the alternative sigma factor gene, botR, is upstream of ha70. Group IV C. argentinese strains also have ha accessory proteins but the ha70 and ha33 genes are inverted relative to the conventional structure. Group II strains (with the exception of bont/B4 containing a ha70-ha17-ha33-botR-ntnh-bont cluster) have orfX3-orfX2-orfX1-p47-ntnh-bont clusters, which lack the botR gene completely; which is also observed in C. baratii F7 and the neurotoxic C. butyricum strains [Citation267,Citation268]. This diversity is likely attributable to recombination events and horizontal gene transfer between strains, which have also led to neurotoxin gene clusters present in non-Clostridium sp. such as Enterococcus sp. 3G1_DIV0629 and Weissella oryzae SG25 [Citation59]. The Weissella neurotoxin gene is present without accessory genes, the ntnh-like gene downstream of bont/Wo lacks significant domains compared to the well conserved ntnh [Citation269]. The insertion elements (IS) and flagellin genes flanking and internal to the gene clusters indicate the probable method of transfer into the strain as it evolved.](/cms/asset/29918186-d3f5-4b59-9072-a8a0cd957d43/kvir_a_2205251_f0007_oc.jpg)
Figure 8. Sporulation and germination of the C. botulinum Groups. The vegetative cell enters the sporulation program when environmental stressors are encountered. Following lysis of the mother cell, the mature spore is released into the extracellular environment, with diverse spore morphotypes. The elucidated spore morphotypes are presented for isolates of Groups I-III. All spore sub-types possess the fundamental spore core (Co), cortex (Cx) and coat (Ct). Exosporium (Exo) present in Group I (thick and loose fitting) and Group III (thin and tight fitting). Appendages (App) present in Group II spore isolates. The diverse germination mechanisms of Groups I-IV: germinant receptors (GR) and core lytic enzymes (CLE).
Data availability statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.