Figures & data
Figure 1. Cyclin dependent kinase 5 (CDK5) suppresses the production of IFN-β.
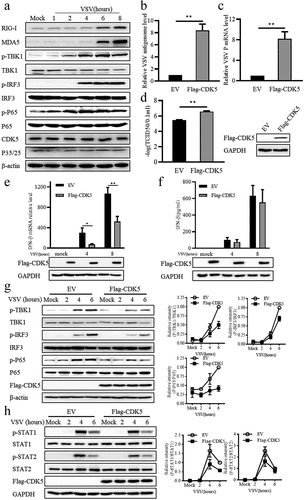
Figure 2. CDK5 deficiency promotes IFN-β production.
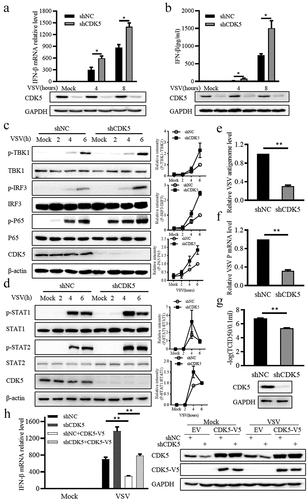
Figure 3. The regulation of CDK5 in IFN-β production is independent of its kinase activity.
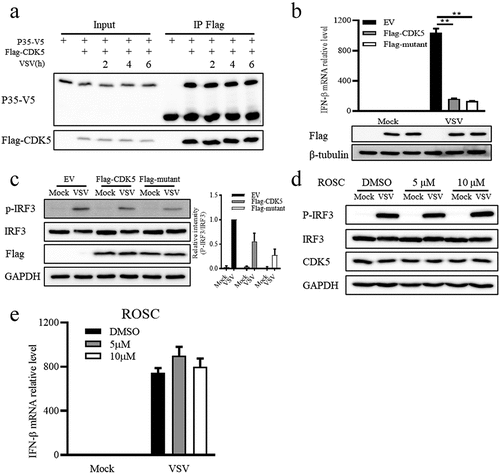
Figure 4. CDK5 attenuates the expression of retinoic acid-inducible gene-I (RIG-I) and melanoma differentiation-associated protein 5 (MDA5).
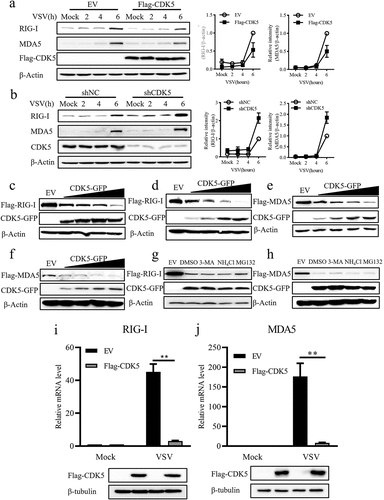
Figure 5. CDK5 is associated with myeloid differentiation primary response gene 88 (MyD88) and suppresses TLR-MyD88-mediated signalling pathways.
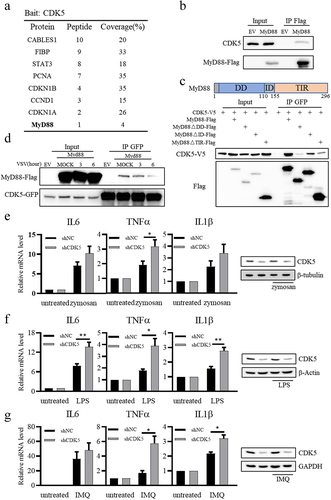
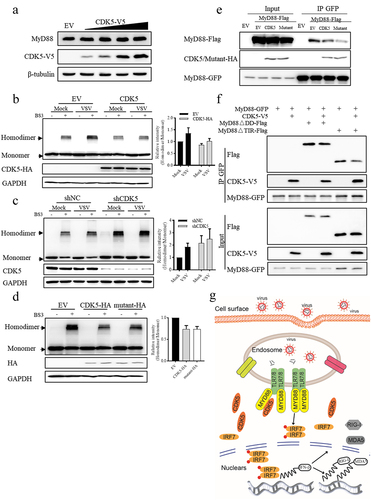
Figure 6. CDK5 negatively regulates the formation of MyD88 homodimers independent of its kinase activity.
Supplemental Material
Download Zip (261 KB)Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materialshttps://doi.org/10.6084/m9.figshare.23512494.
