Figures & data
Figure 1. Identification of isosakuranetin as a dual inhibitor of SrtA and Hla in S. aureus (a) First, SrtA inhibitors were screened from natural products. The FRET method was used to screen inhibitors based on the fluorescence changes in the LPXTG motif recognized and cleaved by SrtA and to screen for SrtA inhibitors (right side of Figure 1A). Subsequently, hemolysis assays were further utilized to finally obtain inhibitors that could inhibit both SrtA and hemolytic activity. (b) The chemical structure of isosakuranetin. (c) Isosakuranetin inhibited SrtA activity in a dose-dependent manner with a calculated IC50 of 21.20 μg/mL. (d) Reversible assay confirmed that isosakuranetin was a reversible inhibitor of SrtA. Untreated SrtA was used as a control. (e) Isosakuranetin suppressed the hemolytic activity of S. aureus USA300 in a dose-dependent manner. n.s. indicates P>0.05; **P < 0.01, *** P < 0.001. The IC50 was determined using the variable slope model in GraphPad, which plots the logarithm of the inhibitor concentration against the normalized response. Hemolysis inhibition rates were rigorously assessed via one-way ANOVA.
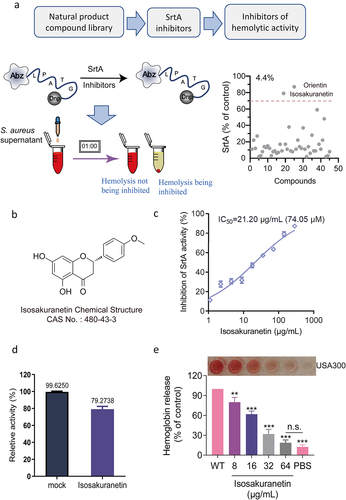
Figure 2. Isosakuranetin does not interfere with the growth of S. aureus USA300 (a) The minimum inhibitory concentration of isosakuranetin against S. aureus USA300 is greater than 512 μg/mL based on the multiplicative dilution method. (b) Growth curves of S. aureus USA300 in the presence or absence of isosakuranetin (32 or 64 μg/mL). The ΔsrtA strain without any treatment served as a control. n.s. indicates P>0.05. The statistical analysis of the MIC data was rigorously conducted using one-way ANOVA.
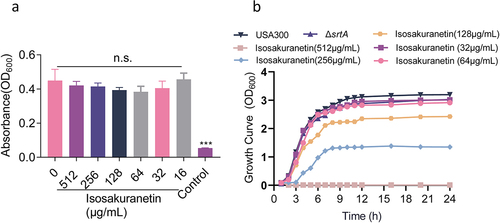
Figure 3. Isosakuranetin inhibition of SrtA and related virulence phenotypes of S. aureus (a) Isosakuranetin inhibited the adhesion of S. aureus USA300 to fibrinogen. (b) Isosakuranetin inhibition of S. aureus USA300 invasion into A549 cells. A549 cells were infected with S. aureus pretreated with different concentrations of isosakuranetin and lysed 2 hours after infection. The invasion of intracellular S. aureus was determined by serial dilution on TSB agar plates. (c) Effect of isosakuranetin on biofilm formation of S. aureus. Biofilm formation was quantified by crystal violet (CV) staining. (d) IgG could specifically bind to S. aureus surface protein (SpA), and FITC-labeled IgG was used to detect the fluorescence change to evaluate the effect of isosakuranetin on the SpA anchoring of the S. aureus cell wall. * P < 0.05, ** P < 0.01, *** P < 0.001. All experimental data depicted in this figure were analyzed using GraphPad, with statistical assessments conducted via one-way ANOVA.
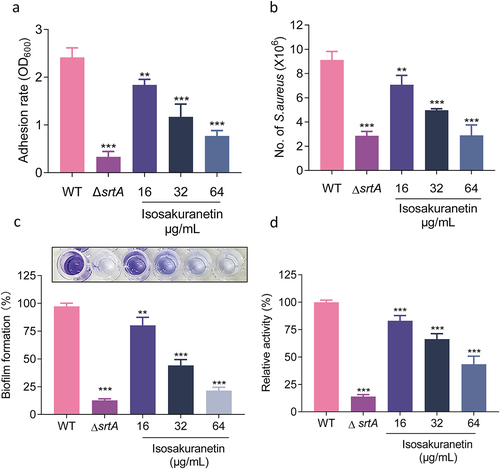
Figure 4. Isosakuranetin regulates the expression of Hla through the agrA system. (a) Isosakuranetin did not affect the neutralizing activity of Hla in S. aureus. (b) Western blotting results showed that isosakuranetin reduced the expression of Hla in the supernatant of S. aureus in a dose-dependent manner. (c) qPCR revealed that isosakuranetin inhibited the transcription of Hla and (d) RNAIII in S. aureus in a dose-dependent manner. * P < 0.05, *** P < 0.001. All experimental data incorporated in this figure were analyzed using GraphPad, with statistical assessments conducted via one-way ANOVA. Grayscale analysis of western blotting results was performed utilizing ImageJ.
Figure 5. Mechanistic study of the interaction between isosakuranetin and SrtA. (a) S. aureus was treated with different concentrations of isosakuranetin, and the expression level of SrtA protein was evaluated by western blotting. ClpP was used as an internal reference. (b) A fluorescence quenching assay was used to evaluate the binding affinity of isosakuranetin to SrtA. (c) Molecular docking of isosakuranetin binding to SrtA and (d) analysis of the major binding amino acid residues and interaction forces of isosakuranetin binding to SrtA. (e) The total binding free energy of isosakuranetin to SrtA was -8.4 kcal/mol. (f) SrtA and SrtA mutants were incubated with 64 μg/mL isosakuranetin, and the activity of recombinant SrtA was determined by FRET. Three independent experiments were used for analysis. The data are presented as the mean ± SD. ***P <0.001 vs. the vehicle or WT-srtA; n.s. represents no significant difference. The western blotting data were statistically analyzed via one-way ANOVA, while the reversible inhibition experiments were examined using Student’s t test.
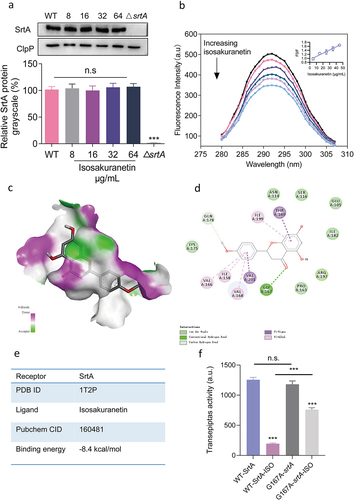
Figure 6. Isosakuranetin protected mice from S. aureus-induced pneumonia. (a) An S. aureus pneumonia infection model was established by nasal drip administration of an S. aureus suspension (2×108 CFUs). Isosakuranetin was administered after infection, and the survival rate of mice within 96 h was evaluated. (b) Determination of the effect of isosakuranetin on the survival of mice (n=10) with S. aureus-induced pneumonia. (c) An S. aureus pneumonia model was established by intranasal administration of an S. aureus suspension (1×108 CFUs). One hour after infection, the mice were treated with isosakuranetin, and the lung bacterial load, wet-to-dry weight ratio, pathological changes and major inflammatory factors were evaluated after infection for 48 h. (d) Effect of isosakuranetin treatment on bacterial load in the lungs of mice (n = 6). (e) Effect of isosakuranetin treatment on the wet to dry weight ratio in the lungs of mice. (f) Histopathological changes (H&E staining) in the lungs of S. aureus-infected mice with or without isosakuranetin (50 mg/kg). (f-h) Effect of isosakuranetin on the secretion of the validation factors TNF-α, IL-1β, and IL-6 in the bronchoalveolar lavage fluid of mice in each group. Animal data were obtained by two experiments (magnification: 400×; scale bar, 50 μm). * P < 0.05, *** P < 0.001. In Fig. 6D, the lower detection limit in our study signifies the absence of discernible bacterial presence. Survival rates were determined using the Kaplan-Meier method, and survival analyses were executed employing the log-rank test. All remaining statistical analyses were rigorously conducted using ANOVA.
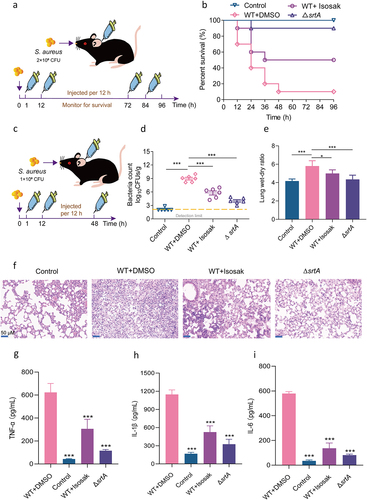
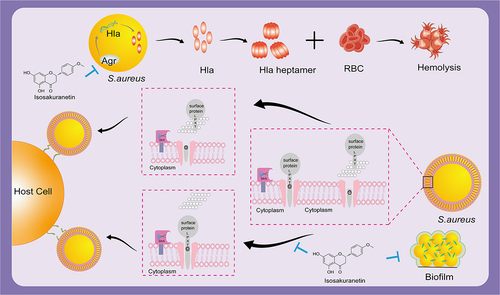
Figure 7. Isosakuranetin reduces S. aureus virulence and fights MRSA infection by inhibiting SrtA activity and interfering with the upstream pathway of Hla. Isosakuranetin is a potent compound that effectively reduces the virulence of S. aureus. It reduces bacterial adhesion to host cells by specifically inhibiting SrtA enzyme activity while inhibiting the formation of S. aureus biofilms. In addition, it disruptively interferes with the pathway upstream of Hla and affects Hla secretion through the Agr system. This mechanism of action illustrates the therapeutic promise of isosakuranetin in the face of MRSA infections, offering a ray of hope for the sustained fight against antibiotic-resistant strains.
Supplemental Material
Download MS Word (65.1 KB)Data Availability statement
The data that support the findings of this study are available from the corresponding author.
