Figures & data
Figure 1. Influences of diaryl chalcogenides on C. albicans SC5314 hyphal formation, biofilm formation and virulence. Effects of diaryl chalcogenides on the hyphal formation (a) and biofilm formation (b) of C. albicans SC5314 at 100 µM. (c) analysis of the effect of compounds and fluconazole (1 µg/mL) on the cytotoxicity of C. albicans SC5314 to A549 cells. Cell cytotoxicity was detected and measured as LDH release. The LDH released by A549 cells in (c) after inoculation with C. albicans in the absence of compounds was defined as 100% and used to normalize the LDH release ratios of other treatments. The compounds were dissolved in DMSO, and the equivalent amount of DMSO was added as a control. Each experiment was performed at least three times in triplicate. Data represent the means ± standard deviation of three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (unpaired t-test).
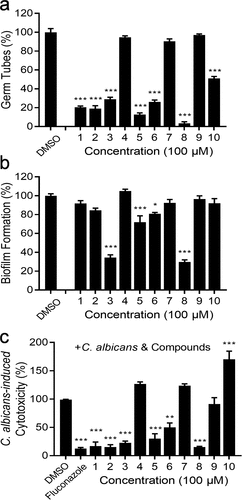
Figure 2. Different concentrations (6.25–100 µM) of compounds 3 and 8 and fluconazole (1 µg/mL) on C. albicans SC5314 hyphal formation (a), biofilm formation (b) and cytotoxicity (c). Data are means ± standard deviations from three independent experiments. ***P < 0.001 vs DMSO (unpaired t-test).
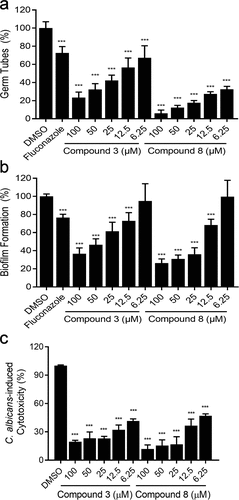
Table 1. Minimum inhibitory concentrations (MICs) of the compounds 3, 8 and fluconazole against the Candida strains and N. glabrata used in this study.
Figure 3. Efficacy of diaryl chalcogenide compound 3 (100 µM) and compound 8 (100 µM) against C. albicans SC5314 in the mouse infection model. (a) survival rate of mice after infection with C. albicans SC5314 without treatment with (●) and by treated with fluconazole (10 µg/mL, ○), compound 3 (▲) and compound 8 (▽). (b) the in vivo pathogen cell numbers of C. albicans SC5314 in the mouse tongue after infection without treatment with and by treated with compound 3 and compound 8 (100 µM). (c) pathological sections were evaluated to determine the effects of compounds 3 and 8 (100 µM) on C. albicans SC5314 infection. The red arrows showed the C. albicans SC5314 infection sites in the mouse tongues. Data are the mean ± standard deviation of three independent experiments. ***, P < 0.001 (unpaired t-test). CA: C. albicans.
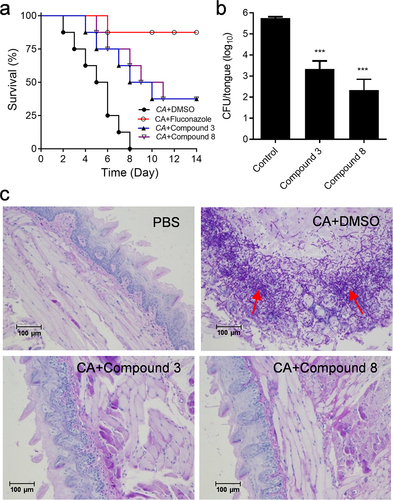
Figure 4. Analysis of biofilm formation (a), cytotoxicity (b) and hyphal formation (c) of different Candida spp. and N. glabrata clinical isolates in the absence or presence of compound 3 and compound 8 (100 µM). Data represent the means ± standard deviation of three independent experiments. **, P < 0.01; ***, P < 0.001 (unpaired t-test).
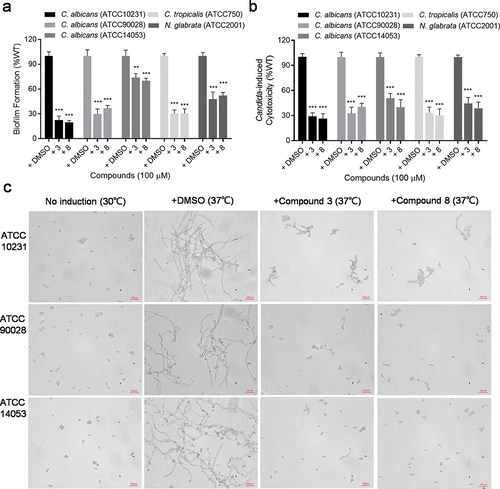
Figure 5. Effects of compounds (100 µM) and their synergistic activities with fluconazole on the biofilm formation (a) and virulence (b) of the clinical drug-resistant C. albicans strain. Data are means ± standard deviations from three independent experiments. **, P < 0.01; ***, P < 0.001 (unpaired t-test).
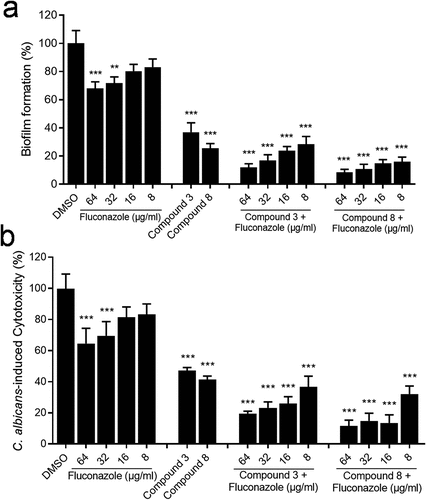
Table 2. Synergistic activities of compounds 3 and 8 with fluconazole, nystatin, and voriconazole against the C. albicans SC5314 wild-type strain.
Table 3. Synergistic activities of compounds 3 and 8 with fluconazole against the clinical drug-resistant C. albicans strain HCH12.
Supplemental Material
Download MS Word (3.3 MB)Data Availability statement
The data presented in this study are available in the supplementary materials.
