Figures & data
Figure 1. Enzootic cycle responsible for maintaining B. burgdorferi in tick populations. Ixodes ticks undergo a three-stage life cycle (larva, nymph and adult), with one bloodmeal per stage. Uninfected larval ticks hatch from eggs and feed on a variety of small mammals that can host B. burgdorferi. The larva takes an infected bloodmeal and moults into a next tick stage, which is a nymph. Nymphs are responsible for transmitting the majority of infections to humans, leading to variety of clinical manifestations. Deer are important hosts for adult ticks but they are not effective reservoirs for the spirochaetes. Engorged females lay thousands of eggs, however, the spirochaetes are not transmitted through the eggs to the larval progeny.
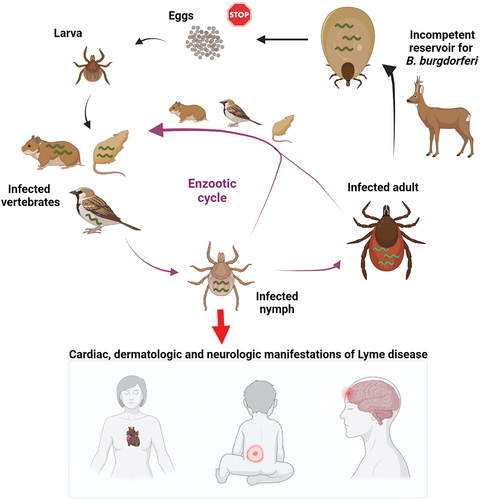
Figure 2. Molecular interactions at the tick midgut luminal interface involved in transmission and acquisition/persistence of B. burgdorferi in ticks. When an infected tick starts feeding on the host, spirochaetes present in the tick midgut multiply, traverse the gut barrier to reach the haemocoel (1), navigate toward the tick salivary glands (2), and ultimately infect the host (3). (a) upon blood intake, the spirochete changes its protein coat to produce molecules such as OspC and BBE31, which enable the pathogen to escape from gut and invade the tick salivary glands. Tick epithelial cells express molecules Ixofin3D and TRE31, which facilitate the process of gut penetration. (b) Borrelia cells ingested in the bloodmeal bind to the tick gut and stay there until a next tick feeding. B. burgdorferi expresses several-exposed lipoproteins such as OspA, OspB and BptA, which protect spirochaetes from bactericidal components present in the host blood and enable them to colonize the tick gut. OspA interacts specifically with the tick receptor TROSPA, thereby enabling efficient colonization of the vector.
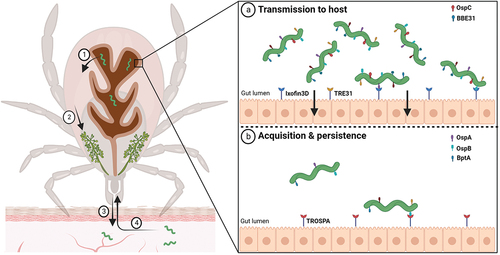
Figure 3. Molecular factors involved in transmission and acquisition of B. burgdorferi by ticks. numerous molecules produced by both a tick vector and Borrelia are required for successful early-stage infection of the host and tick acquisition of B. burgdorferi. Many tick proteins (Salp15, evasins, TSLPI, tHRF, etc.) and borrelial proteins (e.g. OspC, DbpA, DbpB, BBK32) are known to be necessary for transmission. Chemotactic tick protein Salp12 and antioxidant Salp25D, together with borrelial BBA52, were shown to be critical for host-to-tick acquisition of Borrelia.
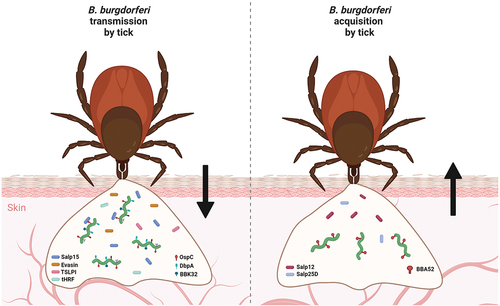
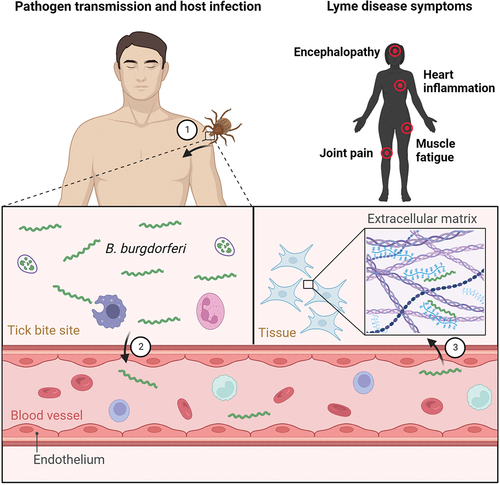
Figure 4. Molecular factors directly involved in active migration and dissemination of Borrelia in humans. (a) B. burgdorferi has to overcome high shear forces generated by blood flow in order to adhere to vascular surfaces for extravasation. The interactions of borrelial protein BBK32 with vascular fibronectin allow active migration in high shear stress environment and borrelial proteins P66 and VlsE aid in vascular transmigration. (b) in low shear stress niches such as extracellular matrix of target skin tissue, borrelial surface-exposed proteins DbpA and DbpB facilitate the translational motion by interactions with matrix molecules such as decorin.
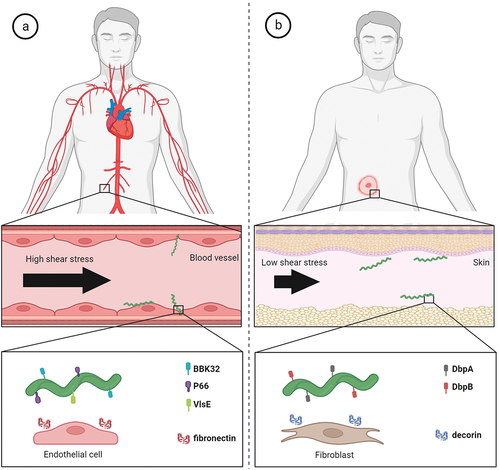
Table 1. Surface proteins of B. burgdorferi which are known to mediate attachment to the extracellular matrix (ECM) components of the vertebrate host.
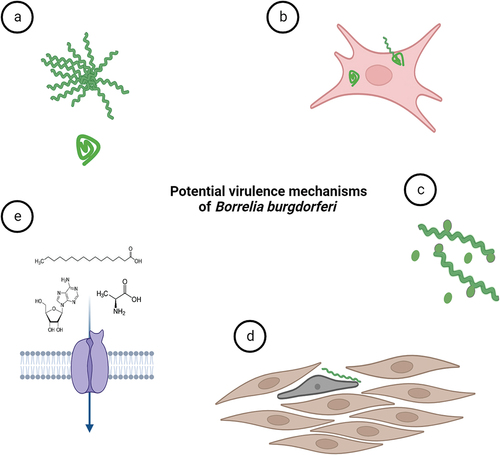
Figure 5. Potential virulence mechanisms of B. burgdorferi. (a) B. burgdorferi can change its morphology as a response to environmental stress. Pleomorphic forms of B. burgdorferi such as biofilm-like structures (or aggregates) and round bodies could possibly help the spirochaetes to overcome prolonged stress conditions exerted for instance by antibiotics. (b) Although B. burgdorferi is considered extracellular parasite, the spirochete is occasionally found inside the nonphagocytic cells. The intracellular niche might help them to hide from immune responses. (c) outer membrane vesicles produced by the bacterium may modulate host immune responses. (d) structural transformation in cell shape and actin cytoskeleton of human cells were shown upon contact with Borrelia. (e) B. burgdorferi relies on uptake of essential nutrients such as amino acids, fatty acids and nucleosides from its host environments for survival and infection. Nutritional virulence might constitute important virulence factors for B. burgdorferi.
Data Availability statement
Data sharing is not applicable to this article as no datasets were generated or analysed in the current study.