Figures & data
Figure 1. Phylogeny of Metarhizium spp. (a) a phylogenomic tree for the Metarhizium species used in this study. Right of tree, the bars indicate times for the onset of a fly’s immune response (detected by Drs-GFP fluorescence), immobilization and death. The % germination provided are after 18 or 40 hrs in yeast extract medium (YEM) or in glucose medium, and after 16 hrs on insect cuticle (fly wings). Fungal growth in the hemolymph (colony forming units (CFU’s) at the commencement of the immobilization period), sporulation (spore counts per cadaver) and radial growth on PDA are provided as measures of growth. Further details are provided in Supplemental tables S1 and S2. (b) rapid germination and growth of M. robertsii 2575 conidia (expressing cherry) compared to M. majus 1946 (expressing GFP) on fly wings (the ungerminated conidia of M. majus are approximately 10 µm long) (c) GFP-expressing M. robertsii 2105 photographed on the abdomen and wings of a fly 24- and 60-hours post-infection. Flies or their wings were visualized with epifluorescence, with filters set to detect GFP or cherry fluorescence.
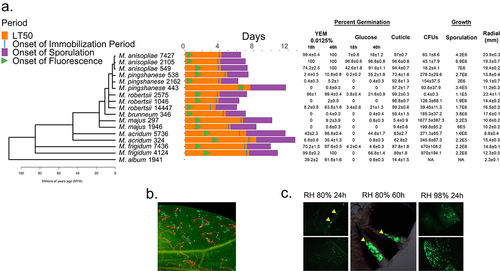
Figure 2. The effect of humidity on Metarhizium spp. Fly (Drs-GFP) immune system responses to infection with 5×106 spores of M. anisopliae 2015, M. acridum 324 and M. pingshaense 443 at different relative humidity’s (98%, and 80%) was studied by measuring drosomycin expression and calculating LT50 values. a) control and infected flies were maintained in petri dishes covered in nylon mesh (pink) and with access to food placed on the mesh. The petri dishes were placed on water saturated tissues (98% RH) or over a saturated solution of NaCl (80% RH). b) faster kills at higher RH elicits earlier and higher Drs-GFP immunofluorescence in infected insects. Fluorescence data was collected 16, 24, 48, and 72 h post-infection. Points represent the means of 10 individual flies±SE. Control flies were treated with water instead of spore suspensions and incubated at different RH in parallel with infected flies.
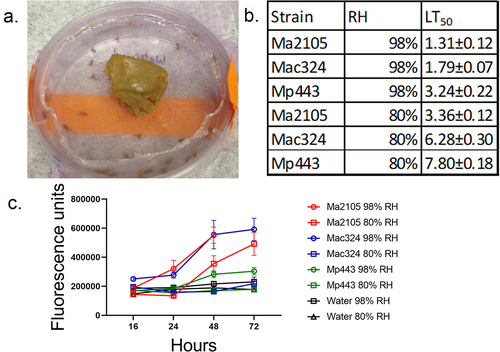
Table 1. The germination rates of two Metarhizium strains on Drosophila melanogaster at different relative humidity.
Figure 3. Drs-GFP immunofluorescence response of D. melanogaster to infection with different Metarhizium strains of high, medium and low virulence to Drosophila.
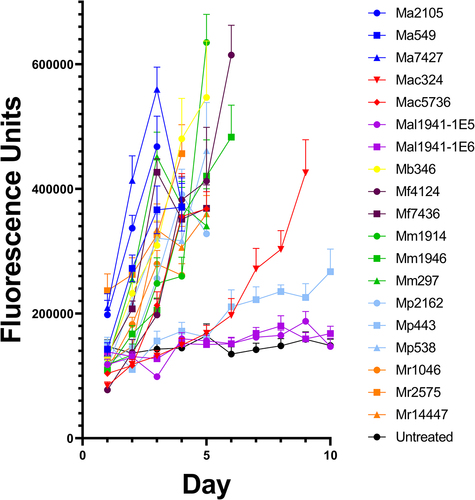
Figure 4. Boxplots showing the variability in immunofluorescence between individual Drs-GFP flies infected with either Ma549 and 2575.
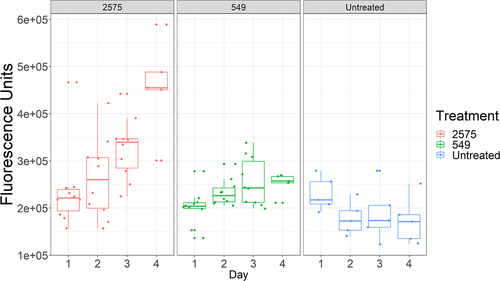
Figure 5. A simplified model of the immune (Toll and Imd) pathways, principally based on Dudzic et al. [Citation38].
![Figure 5. A simplified model of the immune (Toll and Imd) pathways, principally based on Dudzic et al. [Citation38].](/cms/asset/54ddf2ab-f1e6-4c78-888d-2543b9bea58b/kvir_a_2275493_f0005_b.gif)
Figure 6. Quantifying the role that individual components of the insect immune system play in resisting infection. survival (measured as LT50’s) of fly lines disrupted in known immune genes and their isogenic WT backgrounds against M. anisopliae (Ma549), M. frigidum (Mf7436) or B. bassiana (Bb80.2). Shown is the combination of three independent experiments for each pathogen with ~ 20 flies per genotype per experiment. Significance was evaluated using t-tests and is shown relative to the WT (***p < 0.001; **p < 0.01; *p < 0.05).
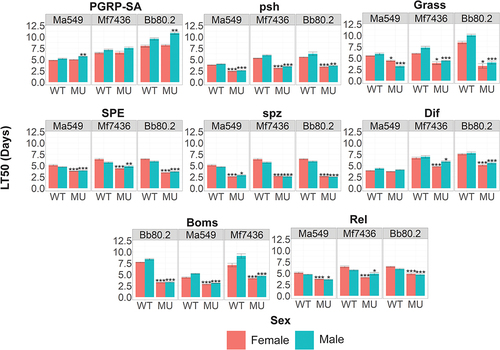
Figure 7. The effect of drosomycin on Metarhizium. (a) percent germination of spores of Metarhizium spp in water or yeast extract plus or minus drosomycin. (b) N. crassa on agar showing growth inhibition by 0.01 µg drosomycin applied to center of plate.
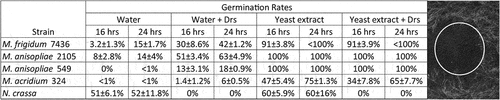
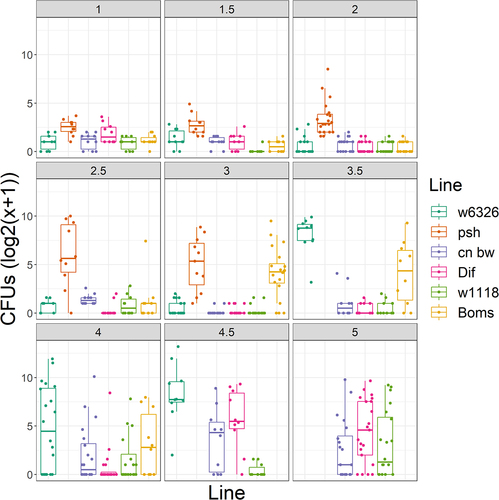
Figure 8. Time course of Ma549 fungal loads in the hemolymph of select immune mutants and their corresponding isogenic WT backgrounds. Results are presented as box plots.
Supplemental Material
Download Zip (150.7 KB)Data Availability statement
The authors confirm that data supporting the findings of this study are available within the article and its supplementary materials.
