Figures & data
Figure 1. Shigella spp. pathogenesis. After ingestion and in its transit towards the intestine, Shigella must resist hostile conditions of the gastrointestinal tract e.g. stomach acidic pH and bactericidal action of bile salts. Once in the gut, Shigella competes with members of the commensal microbiota for space and resources. In the colonic epithelium, M cells transcytose Shigella from the apical to the basolateral side where it is internalized by macrophages. Shigella avoids vacuole-mediated macrophage degradation and induces their inflammatory pyroptotic cell death. Inflammation recruits neutrophils, which are efficient killers of Shigella. However, neutrophil migration damages the epithelial layer. Released from macrophages, Shigella invades epithelial cells through their basolateral side, multiplies intracellularly suppressing immune responses and cell death to extend the life of the infected cell. Bacteria then spread into neighbouring cells through actin-based motility. Finally, Shigella is released from the host through faecal matter.
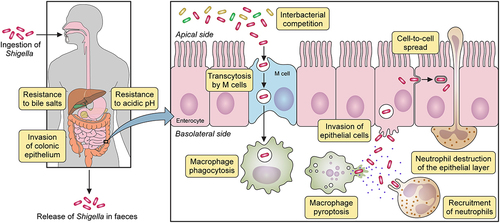
Figure 2. Differences in the interactions with macrophages and epithelial cells between Shigella flexneri and Shigella sonnei. (a) Shigella flexneri (depicted in magenta) shows increased internalization into macrophages mediated by its type 3 secretion system (T3SS), whereas the low internalization of Shigella sonnei (depicted in blue) depends predominantly on phagocytosis. Shigella sonnei also escapes from the shigella containing vacuole less efficiently in comparison to Shigella flexneri, which leads to a reduced cytosolic bacterial count. As a consequence, shigella sonnei induces less macrophage pyroptosis than Shigella flexneri. These differences are attributed to the double layer of O-antigen (found in the LPS and the group 4 capsule) in the cell envelope of Shigella sonnei (b) in contrast to Shigella flexneri infections, characteristic membrane ruffling is not observed upon invasion of epithelial cells by Shigella sonnei. The reduced invasion results in reduced intracellular growth of shigella sonnei compared to Shigella flexneri. Similar to macrophage interactions, these differences are linked to the presence of the double layer of O-antigen in Shigella sonnei.
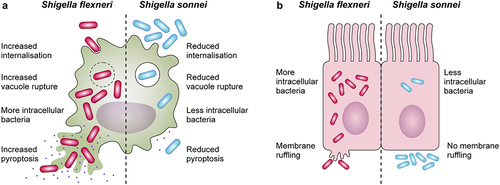
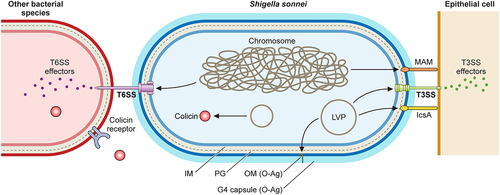
Figure 3. Shigella sonnei virulence factors. The large virulence plasmid (LVP) of Shigella sonnei plays a pivotal role in its virulence since it encodes for adhesin IcsA, the type 3 secretion system (T3SS) and the O antigen (O-Ag) synthesis cluster. The type 6 secretion system (T6SS), adhesin MAM and the capsule export locus are encoded on the chromosome, and colicins are usually encoded on other plasmids. During the invasion of epithelial cells, outer membrane proteins MAM and IcsA act as adhesins that facilitate T3SS-mediated invasion. The T3SS forms pores in epithelial cell membranes that enable the subsequent delivery of effectors that manipulate host cells. The interaction of Shigella sonnei with epithelial cells needs further characterization. Shigella sonnei has also evolved mechanisms to compete with other members of the intestinal microbiota, including a T6SS that requires close contact and colicins that target phylogenetically closely related bacteria and are released after bacterial lysis. Shigella sonnei produces a double O-Ag layer that is present in the LPS and the group 4 capsule and is involved in serum resistance.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.
