Figures & data
Table 1. The role of host vesicular and non-vesicular transport pathway regulators in the nutrient acquisition process of Chlamydia..
Figure 1. Chlamydia hijacks host vesicular and non-vesicular trafficking pathways to acquire nutrients during infection. Inclusion serves as the interface that mediates the interaction between chlamydia and host cells. A series of effector proteins, including inclusion membrane proteins (incs) and T3SS effectors, interact with soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE), ADP-ribosylating factors (ARFs), and Rab GTPases, relocating from fragmented golgi mini-stacks, multivesicular bodies, endoplasmic reticulum, and endosome to acquire nutrients such as sphingomyelin, cholesterol, iron, cation-independent mannose 6-phosphate receptors (CI-M6PR), and transferrin. In addition, chlamydia hijacks host cell non-vesicular transport through incs–CERT interaction to obtain ceramide. Some elements in the figure have been sourced from the SMART database (https://smart.servier.com).
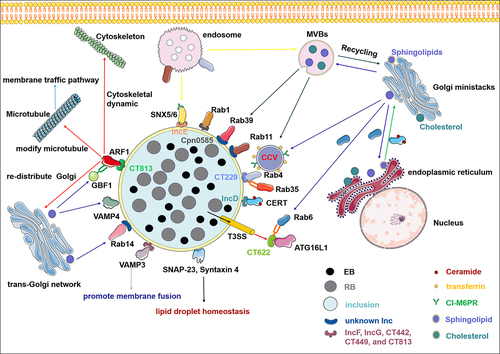
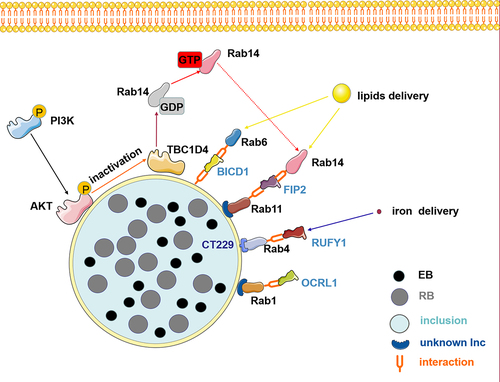
Figure 2. The role of Rab effector proteins and cascade signaling pathways is central to disrupting vesicular transport during chlamydia infection. chlamydia interacts with Rab effectors such as OCRL1, RUFY1, BICD1, and FIP2 to hijack Rab-mediated nutrient transport, redirecting lipid and iron delivery, which are crucial for the development and reproduction of its offspring. Furthermore, chlamydia infection induces AKT phosphorylation and recruits phosphorylated AKT to the inclusion membrane, thus phosphorylating and inactivating TBC1D4. Subsequently, the inactivated TBC1D4 activates Rab14 in a membrane-associated GTP-bound state. Therefore, vesicles marked by Rab14 that contain sphingolipids are redirected toward the inclusion. Elements in the figure are sourced from the SMART database (https://smart.servier.com).
Data Availability statement
Data availability was not applicable to this study. No new datasets were generated in this study and the data cited in this review were obtained from published articles.
