Figures & data
Figure 1. Phylogenetic tree of ONNV and other related alphaviruses. Maximum likelihood phylogenetic tree generated using alphavirus E1 sequences obtained from NCBI databases (GenBank accession numbers included in each sequence name). Sequences were aligned with MUSCLE (v3.8.31) configured for highest accuracy (default settings), ambiguous regions were removed with Gblocks (v0.91b) and HKY85 substitution model used. Only approximate likelihood-ratio tests (aLrts) higher than 0.1 are displayed.
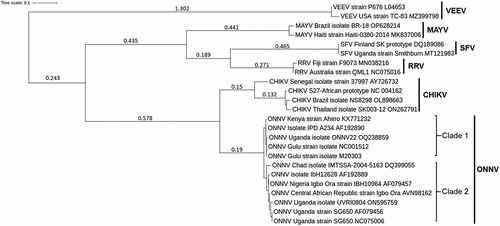
Figure 2. Epidemiology of ONNV. Map depicting African territories with recorded outbreaks of CHIKV (coloured), ONNV (stripped) or both (coloured stripped). Individual studies reporting on ONNV outbreaks or endemic circulation are highlighted in number red dots, with details corresponding to the table provided. CHIKV endemic areas are taken from WHO ([Citation7]).
![Figure 2. Epidemiology of ONNV. Map depicting African territories with recorded outbreaks of CHIKV (coloured), ONNV (stripped) or both (coloured stripped). Individual studies reporting on ONNV outbreaks or endemic circulation are highlighted in number red dots, with details corresponding to the table provided. CHIKV endemic areas are taken from WHO ([Citation7]).](/cms/asset/791598a4-8351-4ece-89d9-843abcedfde4/kvir_a_2355201_f0002_oc.jpg)
Table 1. Reported and confirmed ONNV cases as of 2023.
Figure 3. Overview of ONNV. (a) TEM images of purified ONNV particles taken at 2 different magnifications (courtesy of Malleret, B. and Lee, E.Q.H.). (b) Schematic of the ONNV genome, which consists of a single-stranded positive sense RNA with 2 reading frames, the first encodes nsP1–4, this is followed by a sub genomic sequence that encodes for the viral structural proteins (Capsid, envelope 1–3 and membrane associated 6K protein). (c) Diagram describing the replication cycle of ONNV. Once ONNV releases its genetic material in the host cell, the nsP1–4 proteins are translated, nsP2 then proceeds to cleave the viral polymerase nsP4 which goes on to generate complementary negative RNA strands for rapid viral RNA replication. nsP2 then later cleaves both nsP1 and nsP3 to form the late viral replicase machinery needed for structural protein synthesis and virion assembly utilising host post-translational modifications via ER-golgi apparatus.
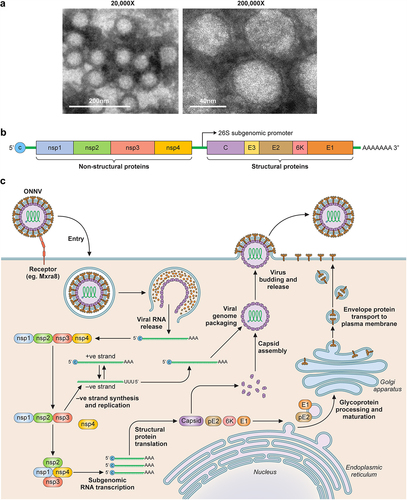
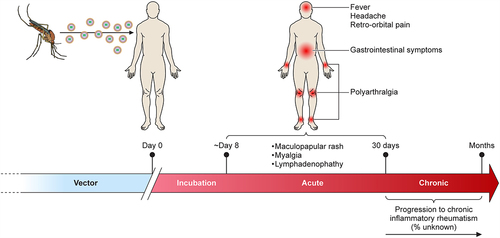
Figure 4. Clinical manifestation of ONNV infection. Diagram showing the general disease progression of ONNV. After being bitten, the virus typically takes ~8 days before the patient becomes symptomatic. This progresses to the acute phase (days 8–15) with generalised symptoms such as fever, rashes, and headaches. In rare cases, ONNV infections will cause lymphoedema and retro-orbital pain, joint pain might also begin to manifest during this time. If not self-limiting, the disease will progress to enhanced pain and inflammation in the joints (targeting extremities) which in rare cases can last for several months (chronic phase).
Data availability statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.
