Figures & data
Figure 1. Medical interventions including surgical wounds and various implanted medical devices associated with increased risk of S. epidermidis and other CoNS opportunistic infections. Created with Biorender.com.
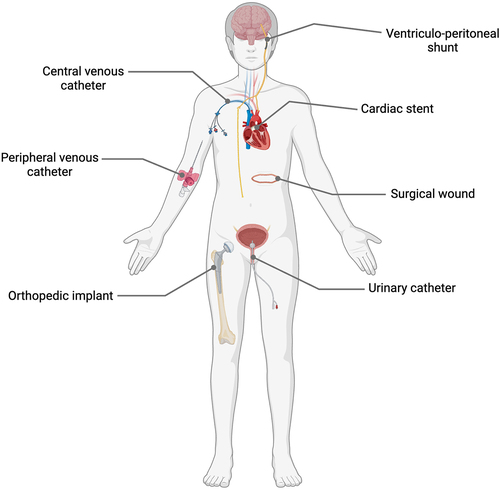
Figure 2. Graphic illustration of the biofilm life cycle in Staphylococcus epidermidis including depictions of important mediators of attachment aggregation, maturation and dispersal stages of biofilm are depicted. The attachment phase is characterised by the deposition of planktonic cells onto a biotic or abiotic surface. This phase is mediated by interactions between host ligands present on the surface, including fibrinogen (Fg) and fibronectin (Fn). Host proteins are then bound by bacterial surface adhesins, such as SdrG and microbial surface components recognizing adhesive matrix molecules (MSCRAMMs), which allow the bacterial cells to remain attached. The accumulation phase results in microcolonies of bacterial cells that self-associate using electrostatic forces and protein adhesins such as accumulation associated protein (Aap). Negatively charged extracellular DNA (eDNA) also surrounds the cells, attracted to the positively charged cell surface and resulting in a “sticky” mesh. A mature biofilm forms when polysaccharide intracellular adhesin (PIA) is excreted by S. epidermidis, forming profuse multi-layered structures. The biofilm matrix shields the bacteria from immune system cells, such as neutrophils, and provides protection from harsh environmental conditions such as desiccation, pH stress and the presence of antibiotics. Channels in the biofilm allow perfusion of nutrients. Within the mature biofilm, a significant proportion of cells remain metabolically dormant, rendering them tolerant to antibiotics that target metabolic processes. The dispersal of biofilm in S. epidermidis is co-ordinated by the action of the agr quorum sensing system, which secretes an autoinducing peptide when cell numbers increase to a critical level. In response to this, transcription of the ica operon, which governs the production of PIA, is reduced and individual cells are released from the biofilm structure. These planktonic cells are then free to disseminate in the host again.
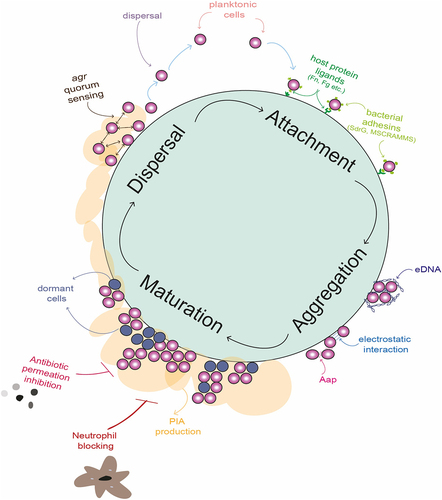
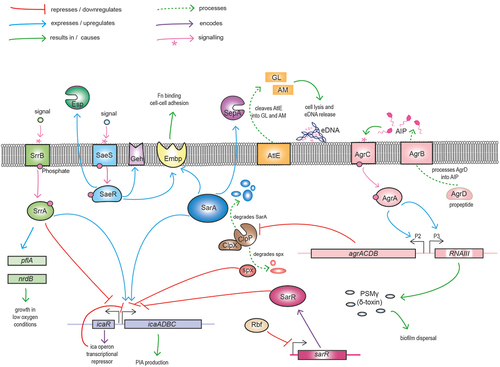
Figure 3. Graphic illustration of major S. epidermidis regulators of growth, biofilm formation and virulence. The icaADBC locus encodes the enzymes responsible for synthesis, export and deacetylation of polysaccharide intercellular adhesin (PIA), which serves as a major mediator of biofilm. The divergently expressed icaR gene encodes the major transcriptional repressor of the ica operon. In response to environmental cues activated SrrB phosphorylates its cognate SrrA effector promoting pflA and ndrB expression and growth under low oxygen conditions. SrrA binds to both the icaA and icaR promoters to differentially regulate biofilm under oxic and microaerobic conditions. Activation of the SaeS kinase leads to phosphorylation of the SaeR response regulator increasing fibronectin (Fn) binding and cell-cell adhesion through upregulation of GehD lipase and the extracellular matrix binding protein (Embp). SaeR also upregulates activity of the serine endopeptidase Esp, negatively impacting S. aureus colonisation and promoting S. epidermidis immune evasion through proteolysis of complement proteins. ClpPX is an ATP-dependent protease and chaperone system that degrades the global regulator SarA, resulting in repression of the repressor icaR and activation of icaADBC and biofilm. Rbf downregulates expression of sarR, which encodes a repressor of the icaADBC operon. The agrD gene from the agrACDB operon encodes a pro-peptide that is exported and processed by AgrB to release the autoinducing peptide (AIP) that constitutes that major quorum sensing system in staphylococci. In response to AIP, the AgrC sensor kinase phosphorylates the AgrA response regulator, thereby leading to activation of the P2 and P3 promoters and increasing expression of the agrACDB and RNAIII loci, respectively. RNAIII is the second effector molecule of the Agr system, functioning as an antisense RNA to control translation of target genes. The cytolysin phenol soluble modulin g (PSMg, δ-haemolysin), encoded by the hld gene located within RNAIII plays a role in biofilm dispersal. The Agr system also negatively regulates expression of the clpP-encoded protease, which degrades Spx, a negative regulator of icaADBC. SarA positively influences Embp expression facilitating cell-cell adhesion. Processing of the major autolysin AtlE by SepA to generate active amidase (AM) and glucosaminidase (GL) autolytic enzymes is important for eDNA release during the early stage of biofilm development.
Data availability statement
The figures in this review are openly available in Figshare (DOIs: 10.6084/m9.figshare.25663989 (); 10.6084/m9.figshare.25092626 () and 10.6084/m9.figshare.25092623 ().
