Figures & data
Figure 1. Illustration of the strategy used to construct chimeric or mutant plasmids. (a) Diagram showcasing the chimeric plasmids pZW-mP9, pZW-mPR187, pYY-rVP3, and the mutant plasmid pZW-g5aa, pYY-5aa(ZW). All these constructs were derived from the parental plasmids pZW and pYY, previously developed in our laboratory, containing the entire genome of the rMDPV strain ZW and the classical MDPV strain YY, respectively. The symbol * denotes the amino acid point mutation introduced into pZW or pYY. (b) Schematic diagram outlining the strategy for constructing chimeric plasmids pZW-mP9 and pZW-mPR187, wherein the P9 promoter or P9-rep sequence was replaced by the corresponding segment from the classical MDPV strain YY. (c) Schematic diagram illustrating the strategy for constructing the mutant plasmid pZW-g5aa or pYY-5aa(ZW) based on the parental plasmid pZW or pYY, involving the introduction of five amino acid mutations through a combination of gene synthesis and overlap PCR methods.
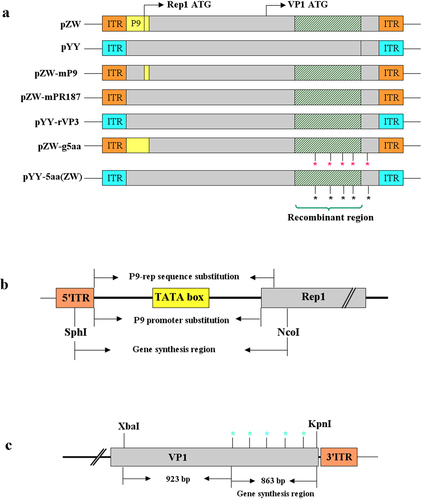
Figure 2. Survival curves and mean death times (MDT) in 11-day-old embryonated Muscovy duck eggs post-transfection or -infection of infectious plasmids or passaged viruses. (a) The survival curve of duck embryos transfected with the mutant plasmid pZW-g5aa significantly differed from those transfected with the parental plasmid pZW (p < 0.001), and the chimeric plasmids pZW-mP9 (p < 0.001), pZW-mPR187 (p < 0.01), and pYY-rVP3 (p < 0.05). However, similar survival curves were observed between duck embryos transfected with the mutant plasmid pYY-5aa(ZW) and the parental plasmid pYY (p > 0.05). No death occurred in duck embryos transfected with the vector plasmid pBSK until 13 days post-infection (dpi). (b) MDT of embryonated Muscovy duck eggs transfected with infectious plasmids. The MDT of pZW-g5aa-transfected duck embryos (199.4 hours) was significantly greater than that of pZW-mP9 (119.1 h) (p < 0.001), pZW-mPR187 (123.7 h) (p < 0.01), pZW (117.5 h) (p < 0.0001), and pYY-rVP3 (136.6 h) (p < 0.01)-transfected duck embryos. Conversely, the MDT of pYY-5aa(ZW)-transfected duck embryos (264 hours) was similar to that of the parental plasmid pYY (224.2 h) (p > 0.05). (c) The first-generation rescued viruses, diluted at 1:50, were individually passaged in 12-day-old Muscovy duck eggs. The MDT of rZW-g5aa (129.2 hours) was significantly greater than that of rZW-mP9 (71.2 h) (p < 0.01), rZW-mPR187 (73.8 h) (p < 0.05), rZW (68 h) (p < 0.01), and rYY-rVP3 (74.2 h) (p < 0.05). Conversely, the MDT of rYY-5aa(ZW)-inoculated duck embryos (144.5 hours) was similar to that of the parental virus rYY (138.5 h) (p > 0.05). Legend: ns, not significant; *p < 0.05; **p < 0.01; ***p < 0.001; and ****p < 0.0001.
Figure 3. ELD50 of the second egg passages of the rescued viruses is compared. The mutant virus rZW-g5aa demonstrates an ELD50 of 105.10/ml, significantly lower than that of the chimeric viruses rZW-mP9 (107.64/ml) (p < 0.01), rZW-mPR187 (107.80/ml) (p < 0.01), rYY-rVP3 (107.90/ml) (p < 0.01), and the parental virus rZW (107.95/ml) (p < 0.001). On the other hand, the mutant virus rYY-5aa(ZW), which carries the five characteristic residues of rMDPV, demonstrates a similar ELD50 (105.65/ml) compared to the parental virus rYY (106.05/ml) (p > 0.05).
Figure 4. Survival curves of 2-day-old Muscovy ducklings infected with the rescued viruses, encompassing three chimeric viruses, two mutant viruses, and two parental viruses. The group infected with the mutant virus rZW-g5aa displayed a significantly distinct survival curve compared to the groups infected with chimeric viruses (rZW-mP9, rZW-mPR187, rYY-rVP3) and the parental virus rZW (p < 0.0001). Conversely, similar survival curves were noted between the group infected with the mutant virus rYY-5aa(ZW) and the parental virus rYY (p > 0.05). Despite witnessing 100% mortality in the groups infected with the three chimeric viruses and rZW, the survival curve of the rZW-mPR187 infection group displayed slight variability compared to the rZW-mP9 and rZW infection groups (p < 0.05), yet similarity to the rYY-rVP3 infection group (p > 0.05).
Figure 5. The pathogenicity and horizontal transmission capability of rYY-rVP3 were assessed using a 6-day-old Muscovy duck model. During the 20-day observation period, the challenge group displayed a 50% mortality rate, with the first death occurring at 5 dpi. In the horizontal contact group, the initial death was observed at 8 days, and cumulative mortality reached 50% within the observation period.
Figure 6. Postmortem examination of deceased Muscovy ducks in the horizontal contact group and viral isolation from tissue samples revealed several pathological signs. These included pericardial effusion (a), fibrous exudates on the liver surface (b), necrotic spots in the pancreas (c), congested kidney (d), and embolism formation in the intestine (e). (f) Liver tissue homogenates from the deceased ducks were used to inoculate 12-day-old embryonated Muscovy duck eggs, resulting in embryo death and the manifestation of hemorrhagic lesions in embryo bodies. (g) Viral DNA extracted from the pooled allantoic fluid was subjected to PCR characterization. Sequence analysis demonstrated 100% homology with the rMDPV strain ZW. Nucleotide numbering was based on the genome sequence of the classical GPV strain LH (accession number KM272560).
Figure 7. Three-dimensional structures and vacuum electrostatics of VP3 are presented. (a) Stereo diagrams illustrate the tertiary structures of VP3 proteins from strain ZW and the mutant virus rZW-g5aa. The mutated residues 252, 350, and 387, likely exposed on the exterior surface of the capsid, are located in two discontinuous peptide chains, which were highlighted in orange and yellow, respectively. (b) Electrostatic surfaces of VP3 proteins from strain ZW and rZW-g5aa demonstrate negative and positive charges represented in blue and red, respectively.
Data availability statement
The data that support the findings of this study are openly available in figshare at http://doi.org/10.6084/m9.figshare.25416529, reference number 25,416,529.
