Figures & data
Figure 1. The ΔD185 and R120X mutant proteins abolish the interaction between RhoGDIα and the Rho-GTPases. HEK293T cells were transiently transfected with a myc-tagged Rho-GTPase and GFP-GDIα (either WT or one of the mutated forms). Cell lysates were immunoprecipitated using anti-myc antibody. Both the precipitates and the total lysates (TL) were immunoblotted for GFP (GDIα). WT GDIα and G173V GDIα co-immunoprecipitated with all 3 Rho-GTPases, but the ΔD185 GDIα and R120X GDIα did not.
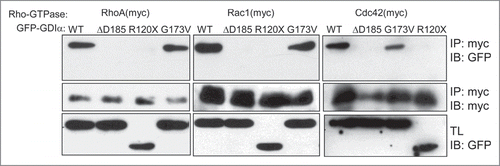
Figure 2. Establishment of GDIα knockdown and GDIα mutant mouse podocyte lines. Mouse podocytes were transduced with control shRNA (control) or GDIα shRNA (GDIα KD) and selected by puromycin. Total lysates were immunoblotted for GDIα, showing effective GDIα KD. The GDIα KD cells were then stably transfected with GFP-tagged WT GDIα (right) or GFP-tagged mutant GDIα (left). The cells with GDIα re-expression were selected with puromycin and G418, lysed and immunoblotted for GDIα. The blot shows effective replacement of endogenous GDIα with GFP-tagged WT or mutant GDIα. The image shown is representative of at least 3 experiments.
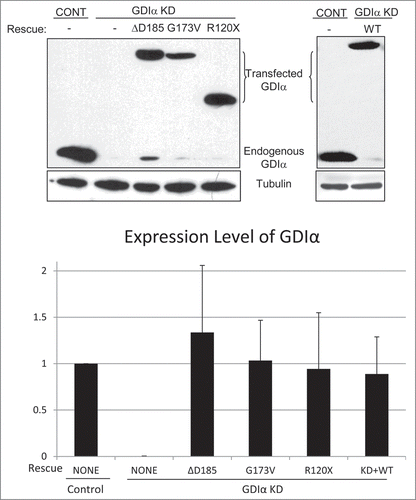
Figure 3. Rac1 remains hyperactivated in presence of mutant GDIα proteins. Active Rac1, RhoA, and Cdc42 were pulled down and immunoblotted in cell lines as shown (A). Bands were quantified by densitometry and normalized to tubulin (as shown in ) and to the control cell line (B). Rac1 activity was consistently increased in the GDIα KD and mutant GDIα cells compared to control cells. This hyperactivity was markedly diminished in cells rescued with WT GDIα, but nonetheless remained significantly higher than control. RhoA and Cdc42 activity were also increased in the GDIα KD cells, but they were decreased to control levels in the cells rescued with WT GDIα. There was a general increase in RhoA and Cdc42 activity in the cells expressing the mutant GDIαs; however, due to great variability, only the RhoA hyperactivity in the ΔD185 GDIα was statistically significant, as compared with control cells. The blots shown are representative of at least 3 experiments. *p < 0.05 vs control, n = 3–6.
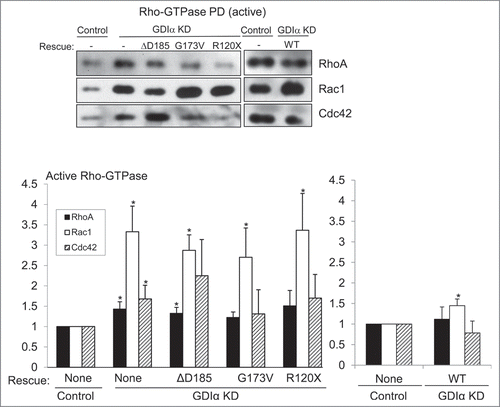
Figure 4. Mutant GDIα proteins do not protect the Rho-GTPases from proteosomal degradation. Western analysis of protein lysates from each mouse podocyte cell line were immunoblotted for Rac1, RhoA, and Cdc42. Total levels of Rho-GTPases were lower in the GDIα KD and the mutant GDIα cells. The blot shown above is representative of at least 3 experiments. Re-expression of the WT GDIα restored Rho-GTPase levels, similar to those in the control levels. Equal loading was confirmed by tubulin. *p < 0.05 vs control, n = 3–6 **p < 0.05 n = 4.
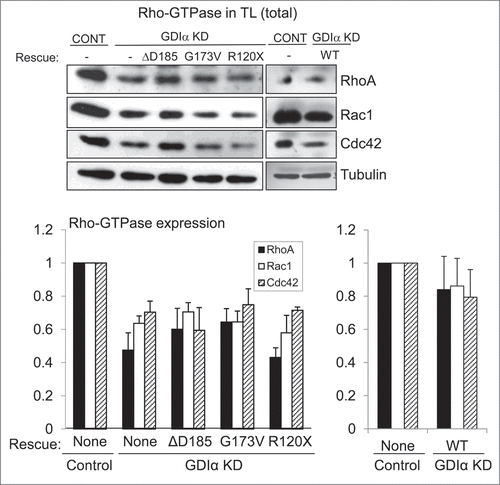
Figure 5. Mutant GDIα podocytes show decreased motility. Wound healing assays (described in Methods) show that the GDIα KD and the mutant GDIα podocytes had decreased motility. Re-expression of the WT GDIα-GFP rescued the GDIα KD cells from this phenotype. Wound healing rate was measured as the fraction of the scratched area which has recuperated. **p < 0.01 vs. control, n = 7–44
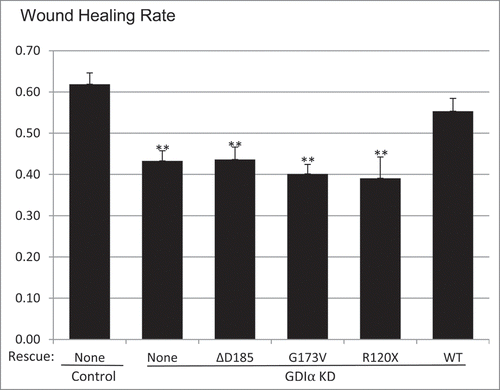
Figure 6. GDIα KD and ΔD185 GDIα podocytes have impaired coordinated movement and overall motility. Cells were stimulated with EGF (100nM) for 3 hours and then tracked by live cell video imaging. (A) (B) and (C) shows the trajectory of control, GDIα KD, and ΔD185 GDIα cells respectively. Axes signify distance from starting point (in μm); different colors represent individual units. (D) shows the total cell displacement and distance traveled over 3 hours in μm. *p < 0.05 vs control n = 12–27 # p < 0.05 vs control n = 3.
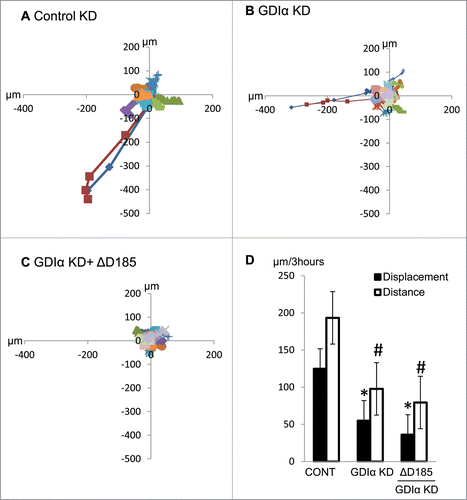
Figure 7. Kymographic analysis shows GDIα KD and ΔD185 GDIα cells have ineffective directional movement. (A) Podocytes were transfected with mCherry-actin and stimulated with EGF (100nM). The pictures show the leading edge of cells over 20 min. (B) Images of the podocytes were captured every 20 seconds over 20 minutes and the total number of phase shifts was counted. The GDIα KD cells had more frequent phase shifts than control cells, reflective of ineffective directional movement. The ΔD185 GDIα cells also had more phase shifts than control; however, the difference was not statistically significant. *p < 0.05 vs control n = 3, 3–4 cells examined per experiment and 1–4 ruffling areas were analyzed per cell.
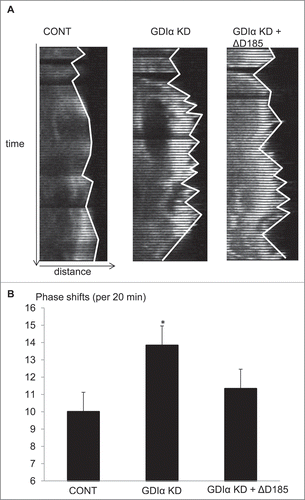
Figure 8. Mutant GDIα podocytes have decreased actin polymerization, smaller cell size, and more cellular protrusions. (A) Actin polymerization was quantified using the ratio of filamentous (F) to globular (G) actin and values were normalized to control. GDIα KD and mutant GDIα podocytes had decreased F/G ratios (n = 60–126 cells from 7 experiments). Re-expression of WT GDIα-GFP rescued the F/G ratio (n = 56–82 cells from 4 experiments). (B) Graphic representation of podocytes' average surface area. GDIα KD and GDIα mutant podocytes had decreased surface area (n = 40–74 from 4 experiments). Re-expression of WT GDIα restored cell size (n = 55–81 cells from 4 experiments). (C) Graphical representation of number of cellular projections. The GDIα KD podocytes had more cellular protrusions than the control podocytes, although the difference was not statistically significant. However, the 3 mutant GDIαs had increased number of cellular protrusions that did reach statistical significance. Re-expression of WT GDIα decreased the number of projections back to control levels. *p < 0.05 vs control **p < 0.001 vs control. Between 49 and 74 cells quantified.
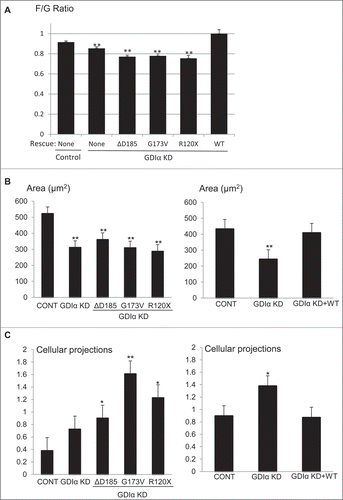
Figure 9. GDIα mRNA expression in podocytes is increased during kidney development. FlexArray analysis of microarray data in podocytes isolated from developing mouse kidneys at embryonic day (E) 13.5, 15.5 and adult kidneys showed that GDIα mRNA increases during development (n = 3 for each stage). *p < 0.01.
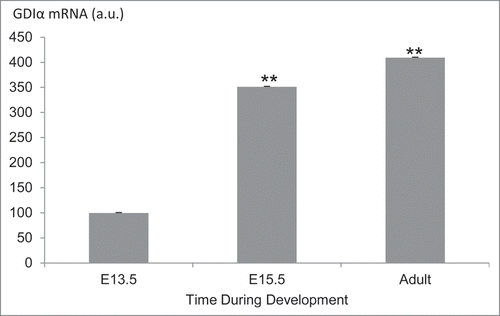
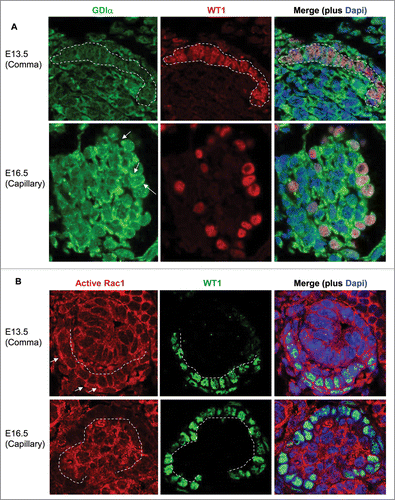
Figure 10. GDIα protein expression increases during kidney development, whereas Rac1 activity decreases. (A) Mouse embryonic kidney sections underwent immunofluorescent analysis with anti-GDIα antibody, WT1 antibody and Dapi. Dotted lines surround podocyte precursors in E13.5 pictures. Arrows show the positive staining for GDIα protein. GDIα protein expression in podocytes was higher at E16.5 than E13.5 (60x magnification). (B) Mouse embryonic kidneys were stained with an anti-WT1 antibody and an antibody that recognizes the active Rac1. There is less active Rac1 at E16.5 than at E13.5. Dotted lines mark the border between podocytes and developing capillaries. Arrows indicate areas of high Rac1 activity at E13.5.