Figures & data
Figure 1. Dual role of Rac1 in cell migration. (A) Schematic representation of the role of Rac1 in promoting cell migration and invasion. Mesenchymal cell migration and invasion is governed by a number of key cellular events, including 1) front-rear polarization characterized by the acquisition of an asymmetrical morphology, reorientation of the nucleus and repositioning of the microtubule organizing center (MTOC) in front of the nucleus; 2) formation of membrane protrusions, including lamellipodia, filopodia and invadopodia; 3) stimulation of focal complex and focal adhesion assembly and turnover; 4) actomyosin contractility to generate the traction force required for cell movement; 5) detachment of the cell rear to allow forward movement of cells; 6) ECM degradation and remodeling through the action of proteases, such as MMPs. Activation of Rac1 is implicated in a number of cellular processes (highlighted in black) that drive cell migration and invasion. (B) Schematic representation of the role of Rac1 in inhibiting cell migration and invasion. Upon activation, Rac1 has been shown to enhance the formation of E-cadherin-mediated cell-cell adherens junctions, which is linked to reduced cell motility and invasion. Additionally, Rac1 also regulates the expression of TIMPs, which counteract the effect of MMPs, thereby inhibiting ECM degradation and reducing cell invasion.
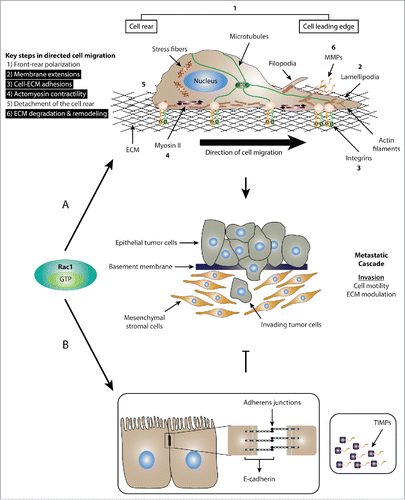
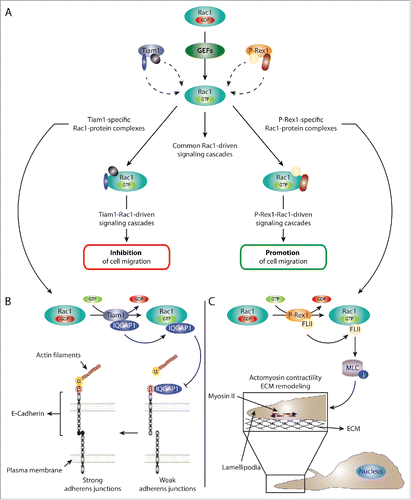
Figure 2. GEFs regulate Rac1 signaling via serving as Rac1 activators as well as scaffolding proteins. (A) Schematic representation of the dual role of GEFs in regulating Rac1 signaling. GEFs activate Rac1 via facilitating the exchange of GDP for GTP. This leads to the association of Rac1 to downstream effectors and the stimulation of various downstream signaling cascades. In addition to acting as Rac1 activators, GEFs can also serve as scaffolding proteins. For example, our data together with evidence from the literature suggest that the scaffolding role of Tiam1 and P-Rex1, 2 Rac-specific GEFs, is important for differentially modulating the Rac1 interactome. As a result, activation of Rac1 by either GEF mediates the formation of a number of GEF-specific Rac1-protein complexes that are important for dictating GEF-specific Rac1 downstream signaling cascades. For simplicity, multiple GEF-specific Rac1-protein interactors are depicted on the same Rac1 molecule; however, it is likely that each GEF drives multiple spatially and temporally distinct Rac1-protein complexes. (B) Schematic representation of the proposed Tiam1-Rac1-IQGAP1 signaling cascade leading to reduced cell migration. Tiam1-mediated Rac1 activation enhances Rac1 binding to IQGAP1. Based on information from the literature, this, in turn, can reduce IQGAP1-β-catenin binding, allowing the formation of stable α-catenin-β-catenin-E-cadherin complexes, thereby leading to stronger adherens junctions. Thus, negative regulation of IQGAP1-mediated cell-cell contact dissociation, might explain the stronger E-cadherin-mediated junctions and reduced cell migration associated with Tiam1-mediated Rac1 activation. (C) Schematic representation of the P-Rex1-Rac1-FLII signaling cascade leading to enhanced cell migration. Through serving as a Rac1 GEF as well as a scaffolding protein, P-Rex1 activates Rac1 while enhancing its interaction with FLII. This leads to increased phosphorylation (depicted by P) of MLC and the activation of myosin II. Given the colocalization of P-Rex1, FLII and actin in lamellipodia, the stimulation of myosin II leads to increased actomyosin contractility and ECM remodeling, potentially at the leading edge. Together, this promotes Rac1-driven cell migration.