Figures & data
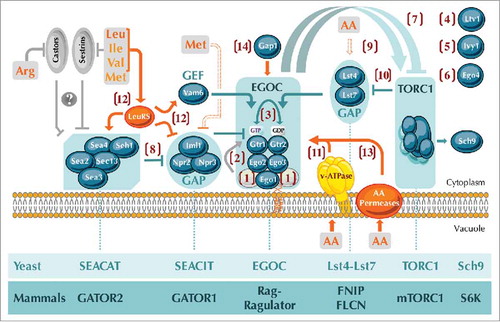
Figure 1. Unsolved questions regarding the amino acid-dependent activation of TORC1 by Rag GTPases and their modulators in yeast. Numbers in brackets refer to the specific questions that remain to be resolved: (1, 2) Is the Gtr1-Gtr2 tethering complex, similar to the mammalian Ragulator, composed of 5 rather than the currently known 3 proteins (Ego1, Ego2, and Ego3) (1), and, if yes, may this potentially pentameric complex exert GEF activity towards Gtr1 (2)? (3) Can the GDP/GTP loading status of Gtr1 impact on the one of Gtr2 and vice versa? (4, 5, 6) What is the role of Ltv1 (4), Ivy1 (5), and Ego4 (6) within the Rag-GTPase-TORC1 network? (7) How do active (i.e. Gtr1GTP-Gtr2GDP) and “inactive” (i.e. Gtr1GDP-Gtr2GTP) Rag GTPases activate and inactivate, respectively, TORC1 in yeast? (8) Does SEACAT negatively regulate SEACIT and, if yes, how? (9) How do amino acids impinge on the Gtr2-GAP complex Lst4-Lst7? (10) Does TORC1 regulate the Lst4-Lst7 complex via a negative feedback loop? (11) Does the v-ATPase mediate vacuolar amino acid signals towards Rag GTPases? (12) By which mechanism does the leucyl-tRNA synthetase signal balanced levels of branched-chain amino acids towards Rag GTPases? (13, 14) Do vacuolar amino acid permeases (13) or the general amino acid permease Gap1 (14) mediate amino acid signals towards Rag GTPases? Yeast SEACAT, SEACIT, EGOC, Lst4-Lst7, and TORC1 complexes have mammalian orthologs coined GATOR2, GATOR1, Rag-Ragulator, FNIP-FLCN, and mTORC1, respectively (lanes below the figure). In addition, the protein kinase Sch9 is the currently best-known bona fide TORC1 target in yeast and is similar to the mammalian TORC1 target S6 kinase (S6K). Color code: grey proteins and arrows/bars refer to mammalian proteins and processes, respectively, while amino acids and focal points that directly sense amino acids are in orange (with the v-ATPase in yellow, as it is currently only known to mediate pH signals towards Rag GTPases). Methionine indirectly prevents the assembly of SEACIT (dashed bar) and specific amino acids directly or indirectly regulate the Lst4-Lst7 complex (dashed arrow). Mammalian Sestrin and Castor proteins sense leucine (and less potently isoleucine, valine, and methionine) and arginine, respectively, and likely (indicated with a question mark) act upstream of the GATOR2-GATOR1 branch. Arrows and bars denote positive and negative interactions, respectively. AA, amino acids; Arg, arginine; Leu, leucine; Ile, isoleucine; Val, valine; Met, methionine; GAP, GTPase activating protein; GEF, guanine nucleotide exchange factor; v-ATPase, vacuolar ATPase. For further details see text.