Figures & data
Figure 1. Targeting Rac1 activation and downstream signaling via blocking Rac1-GEF interactions. (A) Similarly to other Rho GTPases, Rac1 cycles between an inactive guanosine diphosphate (GDP)-bound state and an active guanosine triphosphate (GTP)-bound state. This cycle is regulated, in part, by guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs). Following Rac1 prenylation, in which a geranylgeranyl moiety is covalently attached to cysteine 189, carboxyl-terminal methylation and the addition of a palmitate moiety on cysteine 178, GDP-bound Rac1 associates with the plasma membrane. The Polybasic region (PBR) of Rac1 has also been implicated in targeting Rac1 to the plasma membrane. This, in turn, allows GEFs present at the plasma membrane to bind to Rac1 and facilitate the exchange of GDP for GTP, thereby activating Rac1. As a result of GTP binding, Rac1 undergoes a conformational change in its switch I and switch II regions (depicted as I and II, respectively) that promotes binding with downstream effectors, thus translating upstream signals into downstream responses. In addition to Rac1 activation, GEFs can also serve as scaffolding proteins, via indirectly or directly associating to Rac1 effectors, thereby enriching specific Rac1-effector complexes and dictating Rac1 downstream signaling cascades. It is unclear, however, whether plasma membrane localization is essential for the GEF scaffolding function. In contrast, GAPs serve as Rac1 inhibitors, through enhancing the intrinsic GTPase activity of Rac1 and promoting the hydrolysis of bound GTP. (B) Given the importance of GEFs in activating Rac1 and mediating Rac1 downstream signaling, a number of Rac1 specific inhibitors have been developed that target Rac1 activation via blocking Rac1-GEF interactions. Examples of chemical structures and selectivity toward specific Rac1-GEF interactions are outlined.
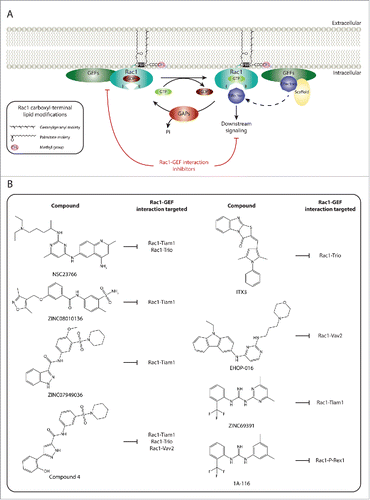
Figure 2. Targeting Rac1 activation and downstream signaling via blocking Rac1-nucleotide interactions. (A) Rac1 is a nucleotide-binding protein that associates with both guanosine diphosphate (GDP) and guanosine triphosphate (GTP). The dissociation of GDP is facilitated by guanine nucleotide exchange factors (GEFs), resulting in Rac1 GTP loading and activation. In contrast, GTPase activating proteins (GAPs) promote the hydrolysis of GTP, thus inactivating Rac1. Following GTP binding, Rac1 undergoes conformational changes in the switch I and switch II regions (depicted as I and II, respectively) that expose the effector binding domain, allowing Rac1 to bind to downstream effectors, thereby mediating downstream signaling. EHT 1864 is a selective Rac1 inhibitor that binds with high affinity to Rac1. This, in turn, promotes the dissociation of nucleotides bound to Rac1, placing Rac1 in an inert and inactive state that is unable to enter the activation GDP-GTP cycle or bind to downstream effectors. (B) The Chemical structure of EHT 1864 and its reported mode of action are outlined.
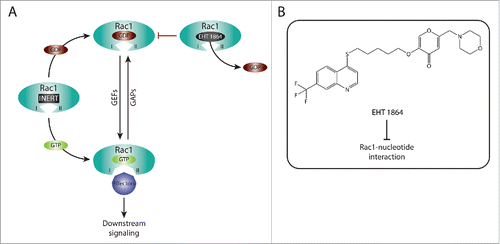
Figure 3. Targeting Rac1 activation and downstream signaling via blocking Rac1 lipid modifications. (A) The isoprenoid pathway is a multi-step chemical cascade, initiated by the 3-hydroxy-2-methylglutaryl-coenzyme A reductase (HMG-CoA reductase). HMG-CoA resides in the endoplasmic reticulum (ER) and represents a rate-limiting step in the conversion of acetyl-CoA into mevalonate, which is converted into isopentenyl diphosphate (IPP), the precursor of geranyl diphosphate (GPP) and farnesyl diphosphate (FPP). Via the action of squalene synthase, FPP can be converted into squalene, the committed precursor for cholesterol production. Alternatively, FPP can be converted into geranylgeranyl diphosphate (GGPP) by GGPP synthase. Both FPP and GGPP can serve as lipid backbones for protein prenylation, which involves the addition of a farnesyl or geranylgeranyl moiety to the cysteine residue within the CAAX motif (where C represents the cysteine, AA represent 2 aliphatic amino acids and X is the terminal amino acid) by farnesyltransferases (FTases) or geranylgeranyltransferases (GGTases), respectively. (B) Following Rac1 protein expression, the CAAX motif (CLLL) located at the carboxyl-terminus is recognized by GGTase type I (GGTase I). This results in the covalent attachment of a geranylgeranyl moiety to cysteine 189. (C) Rac1 prenylation promotes Rac1 translocation and association with the ER membrane where the RAS-converting CAAX endopeptidase (RCE1) and the isoprenylcysteine carboxyl methyltransferase (ICMT) mediate Rac1 post-prenylation modifications on the cytosolic surface of the ER. First, RCE1 cleaves the 3 amino acids (LLL) following the prenylated cysteine residue. This exposes the carboxyl-group of the cysteine amino acid (COO−), thus allowing the transfer of a methyl group (CH3) from S-adenosyl methionine (AdoMet) to Rac1 by ICMT, releasing S-adenosyl- L homocysteine (AdoHcy) as a by-product. The fully processed prenylated Rac1 protein can then be trafficked to the plasma membrane. Rho guanine nucleotide dissociation inhibitors (RhoGDIs) have been implicated in mediating Rac1 extraction from the ER membrane, with the RhoGDI-Rac1 association being tightly regulated and reversible, thus allowing cycling between Rac1 membrane/nuclear translocation and cytoplasmic sequestration. Rac1 prenylation and processing also primes Rac1 for palmitoylation, in which a 16-carbon fatty acid palmitate is attached to cysteine 178 by palmitoyl acyltransferases (PATs). Rac1 palmitoylation also depends on the polybasic region (PBR) within the carboxyl-terminus of Rac1. It is unclear whether palmitoylation of Rac1 occurs in the cytoplasm or on the plasma membrane. It is also yet to be determined whether palmitoylation preferentially occurs on GDP-bound or GTP-bound Rac1, for simplicity the latter was not depicted in the figure. Rac1 palmitoylation enhances Rac1 stability and mediates Rac1 localization to the plasma membrane, particularly to lipid-ordered subdomains, as well as nuclear translocation. Statins, GGTase I inhibitors (GGTIs) and PAT inhibitors have been shown to target HMG-CoA reductase, GGTase I and PAT enzymes, respectively. As a result, all 3 classes of compounds have been shown to disrupt Rac1 localization, activation and downstream signaling.
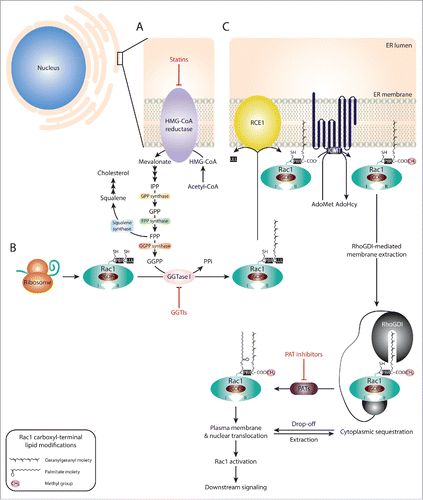
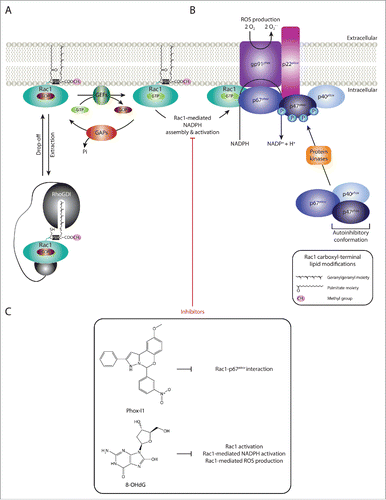
Figure 4. Targeting Rac1-mediated assembly and activation of NADPH oxidases and ROS production. (A) Guanine nucleotide exchange factors (GEFs), GTPase activating proteins (GAPs) and Rho guanine nucleotide dissociation inhibitors (Rho GDIs) regulate Rac1 cycling from an inactive guanosine diphosphate (GDP)-bound state to an active guanosine triphosphate (GTP)-bound state. RhoGDIs also play a role in Rac1 cytoplasmic sequestration. (B) Nicotinamide adenine dinucleotide phosphate (NADPH) oxidases are multimeric protein complexes that transfer electrons from intracellular NADPH to extracellular molecular oxygen, generating superoxide anions (O2.−), a reactive oxygen species (ROS), in the process. Rac1 has been shown to regulate the assembly and activation of NADPH oxidases. For simplicity only one NADPH oxidase isoform, NADPH oxidase 2 (NOX2), is depicted. Activation of the complex involves the assembly of the cytosolic regulatory proteins (p67phox, p40phox and p47phox) with the membrane-associated components (the catalytic subunit gp91phox and p22phox). This is mediated through the phosphorylation of the autoinhibitory region of p47phox, thereby releasing the autoinhibitory conformation and promoting p47phox-p22phox interaction. Additionally, activated Rac1 has been shown to directly bind to the p67phox subunit, thereby further facilitating complex assembly. Although not depicted in the figure, lipid metabolism within the plasma membrane also plays an important role in providing the anchoring sites for p40phox and p47phox. (C) Given the role of Rac1-mediated NADPH assembly and activation and ROS production in the progression of a number of human diseases, inhibitors that specifically target this Rac1 downstream signaling cascade have been identified. Phox-I1 represents a Phox-I class inhibitor that functions via blocking the interaction between Rac1 and p67phox, thereby inhibiting Rac1-mediated complex assembly. Another example includes 8-hydroxy-2-deoxyguanosine (8-OHdG), which also inhibits Rac1-medaited NADPH activation and ROS production. The chemical structures and mode of action of both compounds are outlined.