Figures & data
Figure 1. Diaphanous formins are effectors of the small GTPase signaling. (A) Schematic diagram of the general structure and the mode of action of diaphanous formin proteins. GBD – GTPase binding domain; DID – Dia interacting domain; FH – Formin homology domain; DAD – Dia auto-inhibition domain. Small GTPase binding releases the auto-inhibitory interaction between the DID and the DAD domains. The FH1FH2 alone can be used as a constitutively active construct that functions without the GTPase signaling. (B) Comparison between the 3 members of diaphanous subfamily formins, with emphasis on the cognate upstream small GTPase signaling and the subcellular localization. Scale bar, 10 µm.
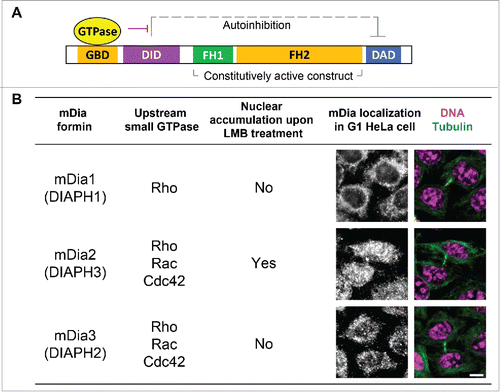
Figure 2. High resolution quantitative live-cell imaging for systematically measurement of total CENP-A levels at centromeres in real time. Left: overlaid plots showing ratiometric measurement of YFP-CENP-A levels from centromeres in multiple cells (light blue/red circles are raw data points, and dark blue/red lines are sample averages). Right: 2 representative frames of cells with YFP-CENP-A on the centromeres pseudocolored based on intensity levels. (Data were replotted from Liu and Mao., JCB, 2016Citation30 Scale bar, 10 µm.).
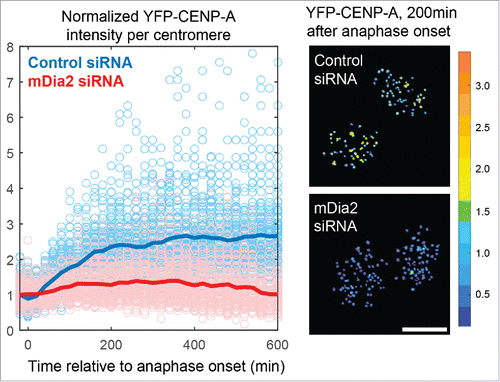
Figure 3. Schematic summary showing mDia2 functions downstream of the MgcRacGAP-dependent GTPase pathway to maintain CENP-A levels at the centromere. Depending on the GEF (Ect2) and GAP (MgcRacGAP), the Rho family small GTPase cycles between active form and inactive form, which is important for CENP-A maintenance at the centromeres. Diaphanous formin mDia2 has an epistatic relationship downstream of the MgcRacGAP-dependent small GTPase molecular switch and promotes G1 CENP-A loading/maintenance.