Figures & data
Table 1. Direct partners of exocyst for migration/invasion functions.
Figure 1. The protein-protein interaction network of Ral-exocyst controlling migration and invasion. The exocyst, a major effector of Ral proteins, directly interact with several key regulators of protrusion formation (Wave Regulatory Complex, Arp2/3 complex, SH3BP1, paxillin), front-rear polarity (Par complex) and extra-cellular matrix degradation (WASH, IQGAP) or deformation (GEFH1), to drive cell migration and invasion.
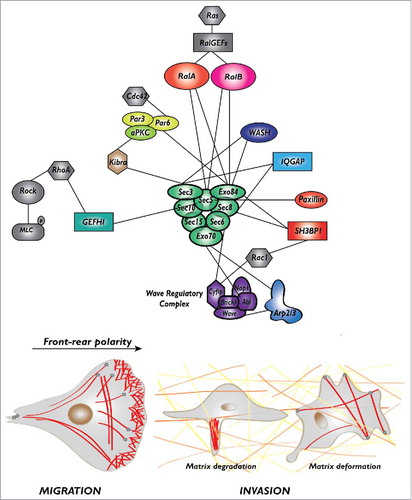
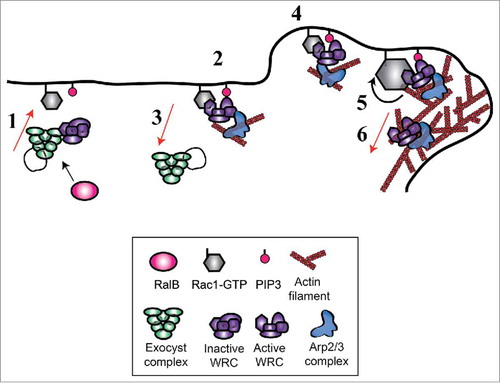
Figure 2. The cross-talk between RalB and Rac1 pathways during cell migration. While both RalA and RalB may participate to control cell migration, here we indicate RalB because its role is more robust and universal. RalB regulates assembly and localization of exocyst complex which in turn directly binds to a negative Rac1 regulator, the RacGAP SH3BP1, and to a major Rac1 effector, the Wave Regulatory Complex (WRC). RalB, exocyst, SH3BP1 and WRC, they all localize at the cell front in migrating cells even though the features and dynamics of their translocations appear different and need more investigation.
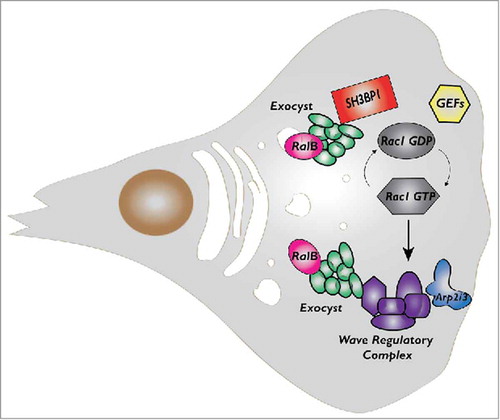
Figure 3. Model for Wave Regulatory Complex (WRC) recruitment and activation. Via interaction with exocyst, WRC is recruited to the front plasma-membrane (1) where it is moderately activated by the existing Rac1-GTP and PIP3 molecules (2). Rac1-GTP retains WRC at the edge, exocyst dissociates from WRC and recycles in the cytosol (3). This initial WRC activation is sufficient to start protrusion (4). The positive feedback loop between actin filaments and Rac1Citation46,Citation47 leads to sustained Rac1 activation and stronger WRC stimulation (5). During the protrusion progression, a fraction of the WRC complex is incorporated in the actin network to be recycled (6).