Figures & data
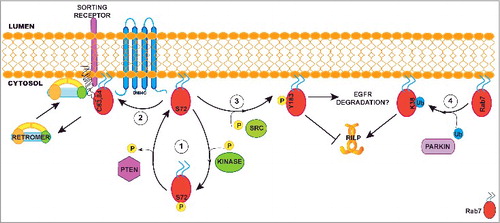
Figure 1. Role of post-translational modifications in modulating Rab7 functions. (1) Phosphorylation on Serine 72 by a non-identified kinase displaces Rab7 from the membrane to the cytosol. Here, the phosphatase PTEN can remove the phosphorylation on Serine 72 enabling Rab7 to bind the membrane again. (2) On the membrane, Rab7 can be palmitoylated by a yet unknown palmitoyltransferase. Palmitoylation on cysteines 83 and 84 is required to recruit retromer at the late endosome to mediate sorting receptor retrieval to the trans-Golgi Network. (3) Src mediated phosphorylation of Y183 blocks Rab7 interaction with its effector RILP. The role of this modification on EGFR degradation is controversial as some groups found that phosphorylation is required for degradation while other found it must not be phosphorylated. (4) Ubiquitination of K38 mediated by the E3-ligase parkin stabilizes Rab7 on the membrane and is required for the interaction with the Rab7 effector RILP. In this schematic view, Rab7 is represented with one post-translational modification at time, but we cannot exclude the possibility that palmitoylation, phosphorylation and ubiquitination are mutually exclusive. In fact, it is feasible that various combinations of post-translational modifications determine the specific function of Rab7 on late-endosomal membranes.