Figures & data
Figure 1. Signalling pathways in the primary cilium. Schematic diagram demonstrating some of the signalling pathways transduced by the primary cilium. Mechanical and chemical extracellular signals are sensed by proteins in the membrane of the cilium, which are then transduced into the cell to activate a variety of intracellular pathways. Figure adapted from [Citation32]. Hh, Hedgehog; PCP, planar cell polarity; PDGFα, Platelet Derived Growth Factor Subunit alpha; Shh, Sonic Hedgehog
![Figure 1. Signalling pathways in the primary cilium. Schematic diagram demonstrating some of the signalling pathways transduced by the primary cilium. Mechanical and chemical extracellular signals are sensed by proteins in the membrane of the cilium, which are then transduced into the cell to activate a variety of intracellular pathways. Figure adapted from [Citation32]. Hh, Hedgehog; PCP, planar cell polarity; PDGFα, Platelet Derived Growth Factor Subunit alpha; Shh, Sonic Hedgehog](/cms/asset/fa197c2a-eb51-44a6-886c-82d679994495/ksgt_a_1703466_f0001_oc.jpg)
Figure 2. Proposed interaction and function of ARL3 in the cilium
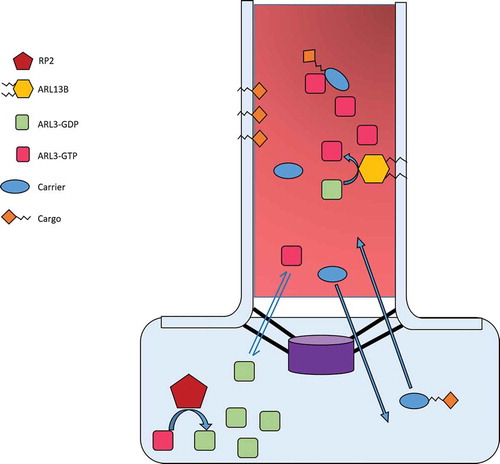
Figure 3. ARL3 missense mutations at a conserved site affects the interaction of ARL3 with ARL13B
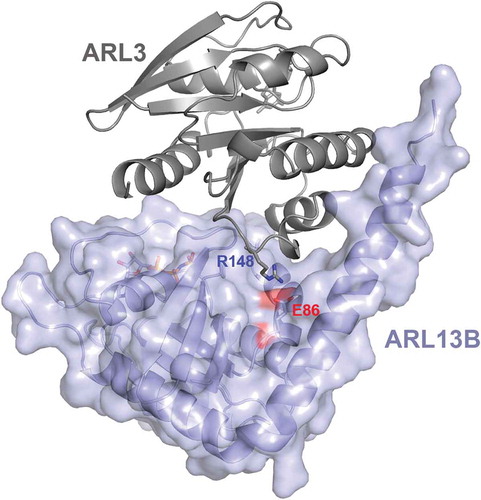
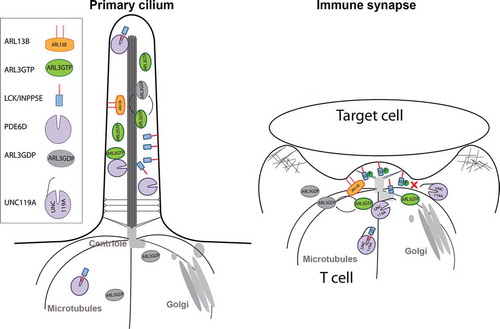
Figure 4. A common role for ARL3, ARL13B and UNC119A in the primary cilia and the immunological synapse