Figures & data
Figure 1. Schematic representation of some of the GEFs discussed in this review showing intra-domain interaction rendering them in autoinhibitory states. (a) GTPase switching mechanism between ON and OFF state. Canonical GEFs such as (b) p115-RhoGEF and (c) PDZ-RhoGEF are proposed to exhibit autoinhibition due to intra-domain interaction involving their RH domain. Similarly, autoinhibition of (d) AKAP-Lbc and (e) p114-RhoGEF is due to intra-domain interaction involving its non-RH, C terminal region. (f) In p63RhoGEF and (g) Vav GEF, the PH domain is suggested to be responsible for its autoinhibition. (h) In P-Rex GEF, the C terminal domains like IPI4, PDZ1, and PDZ2 are responsible for intra-domain interactions. (i) In TIAM1, the initial 50 amino acid domain (N50), N terminal PH domain (PHn) and the coiled coil extension (CC-Ex) domain of TIAM1 interact with each other to render the GEF to its autoinhibitory state. (j) In non-canonical GEFs like DOCK proteins interaction between N terminal SH3 domain and C terminal domains and/or DHR2 domain is responsible for their autoinhibition.
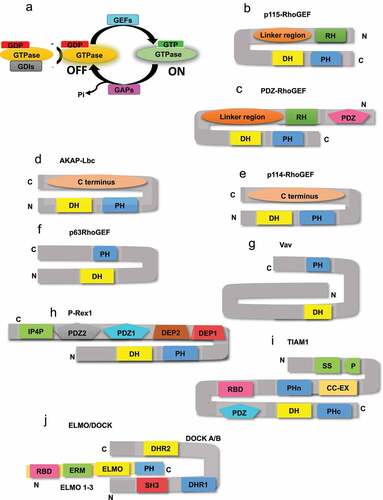
Table 1. Overview of canonical and non-canonical GEFs discussed in this review, their downstream Rho GTPases, tissue-specific expression and activating GPCRs
Figure 2. Schematic representation of signalling pathways which leads to activation of GEFs through interaction between heterotrimeric G protein subunits and RH domains of GEFs. (a) GPCRs, Mas receptor and M1-mAChR activate the GEF, LARG, through Gαq binding to its RH domain. (b) Activation of LARG through GPCRs, LPAR & S1P2R occur through direct interaction with Gα12/13 whereas, GPCRs, G2A & PAR activate LARG through Gα13 subunit. (c) PDZ-RhoGEF mediated RhoA activation is established by GRPR, LPAR, and CXCR4 triggered Gα13 heterotrimeric subunit binding to the RH domain of PDZ-RhoGEF. (d) GPCRs, LPAR, CXCR4, and S1P2R activate p115-RhoGEF through interaction with Gα13 heterotrimeric subunit. (e) Upon activation of β2AR, p115-RhoGEF is triggered by β-arrestin activation through Gαiβγ signalling.
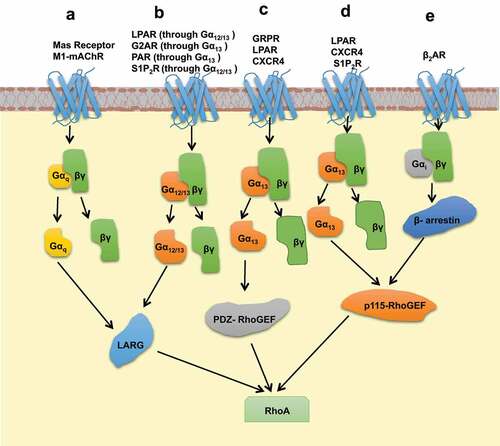
Figure 3. Schematic representation of signalling pathways which leads to activation of GEFs through interaction between heterotrimeric G protein subunits and non-RH domains of GEFs. (a) Stimulation of prostaglandin receptor activates Gαs, which in turn binds to PDZ-RhoGEF through DH, PH domains, and their linker region of GEF, thus making it active towards Cdc42. (b) AKAP-Lbc dependent activation of RhoA, by LPAR and AT1R, happens through the interaction of Gα12 with the C terminus of AKAP-Lbc. (c) and (d) Activation of Gβγ (through M3R and LPAR) and Gα12 triggers p114-RhoGEF, where Gβγ interacts with the C terminus of DH-PH domain of p114-RhoGEF. (e) p63RhoGEF mediated triggering of RhoA upon activation of M3R and H1R receptors. This involves the interaction of Gαq/11 towards the PH domain of p63RhoGEF. (f) Stimulated GPCRs, LPAR, and CXCR4 activate P-Rex1 through the interaction of the Gβγ subunit with the IP4P domain and the tandem PDZ domains of P-Rex1. This may further lead to the activation of ELMO/DOCK2 through P-Rex activated RhoG. (g) Activation of ET1R triggers βPIX mediated Cdc42 through the interaction of Gαq with βPIX.
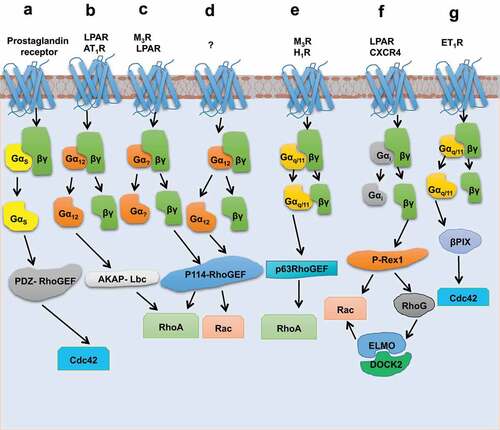
Figure 4. Activation of GEFs through Phosphorylation, protein-protein, and lipid-mediated interactions involving downstream of GPCR signalling. (a) Upon LPAR stimulation, activation of βPIX takes place through, Gαi mediated Src kinase activation, which further phosphorylates βPIX. (b) Activation of fMLPR triggers Cdc42 through Gβγ-PAK1-αPIX and Rac1 through Gβγ-PAK1-αPIX-GIT2 sequential complex formation, where activation of αPIX takes place through PAK1 binding. Cdc42 and Rac1 are involved in feedforward mechanism where αPIX and Rac1 enhance PAK1 activity. Here activation of Cdc42 enhances GEF activity of αPIX towards Rac1. (c) CXCR4 activates Vav1 and Vav2 through JAK signalling. (d) Activation of Rac through fMLPR stimulation involves concerted signalling between Vav1/3 and P-Rex GEFs. (e) LPAR triggers Rac1 through PKC and CamKII mediated phosphorylation of TIAM1. (f) Stimulation of GPCRs, LPAR and S1P1R, activate TIAM through Gαiβγ/PI3K/TIAM1/Rac1 pathway, where TIAM1 interacts with a PI3K lipid product, PIP3. (g) Protein–protein interaction mediated triggering of RhoA when activated Frizzled7 recruits Dishevelled (Dvl) and Daam-1 proteins which activate the Rho WGEF.
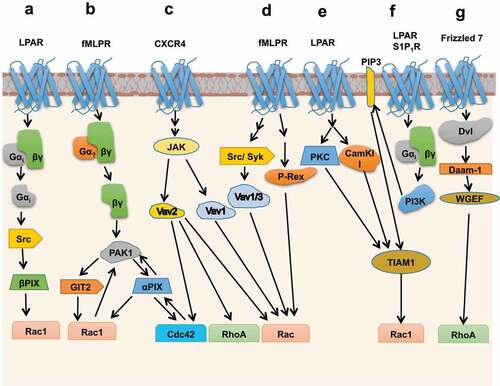
Figure 5. Schematic representation of activation of GEFs upon direct interaction with GPCR domains. (a) GEFs, LARG, and PDZ-RhoGEF activate RhoA signalling upon interacting with the C terminus of LPAR through their PDZ domains. (b) Similarly, Vav2 interacts with the C terminus of PTHR through its DH and PH domains.
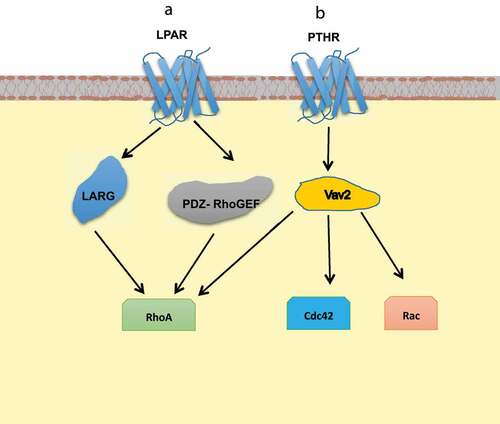
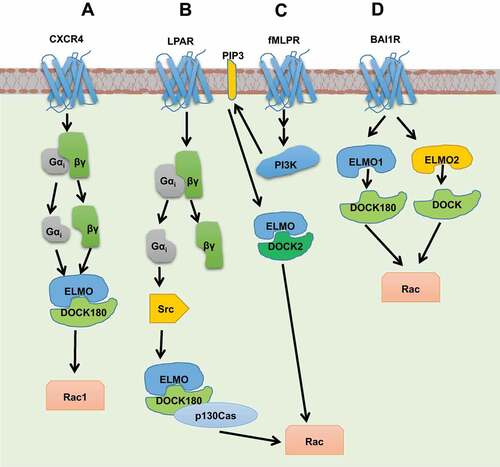
Figure 6. Schematic representation of activation of non-canonical GEFs by GPCRs. (a) CXCR4 activates ELMO1/DOCK180 through the interaction of ELMO1 with Gβγ and Gαi subunits. (b) Stimulation of LPAR leads to Rac activation through Gαi2-Src-p130Cas/ELMO/DOCK180/Rac1 pathway during which Src is proposed to phosphorylate DOCK180 at Tyr1811. (c) ELMO/DOCK2 mediated Rac activation takes place through binding of DHR-1 domain of DOCK2 to lipid, PIP3 upon fMLPR activation. (d) ELMO1 and ELMO2 are shown to interact with the C terminal of the BAI1 receptor through their N-terminal fragments to trigger the DOCK/Rac pathway.