Figures & data
Figure 1. KO mice have splenomegaly and increased BCL-6 expression in B cells compared with WT mice; and RhoH re-expression decreases proliferation of KO mouse cells with lymphoma characteristics. (a) Survival curves of WT and KO mice, n = 66 and 65 respectively. **p < 0.01, Log-rank test. (b) Spleen weights of WT and KO mice under 12 months-age, n = 23 and 12, respectively. (c) Photomicrograph of representative sections from spleens of age-matched WT and KO mice stained with haematoxylin and eosin (H&E) (20X magnification); and immunohistochemistry stains with anti-CD19, anti-BCL-6, and anti-Ki-67 antibodies (20X; inset: 100X magnification). Scale bars are 100 µm. (d) Percentage of Ki-67 positive cells in the white pulp areas of WT spleen versus KO spleen as determined by immunohistochemistry (each value is an average of the percentage of Ki-67 positive cells calculated in 5 different areas in each sample; n = 4). (e) In vitro growth of KO CD19+ B cell line transduced with RhoH-expressing retrovirus vector (KO-RhoH) versus empty vector (KO-Control), n = 4. Data show mean ± SD. * p < 0.05, *** p < 0.0001.
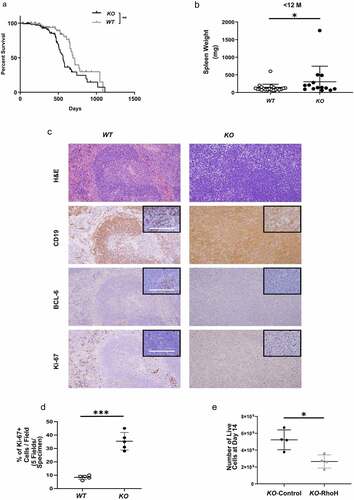
Table 1. Median survival of WTnTg, WTTg, and KOTg mice
Figure 2. KOTg mice show significantly shorter lifespans, increased splenomegaly, and increased lymphoma cell proliferation compared with WTTg mice. (a) Survival curves of WTnTg, WTTg, and KOTg mice, n = 82, 67, and 69 respectively. ** p < 0.01, *** p < 0.001, Log-rank test. (b) Representative photographs of spleens of age-matched WTnTg, WTTg, and KOTg mice. (c) Spleen weights of WTTg and KOTg mice with lymphoma at pre-terminal stage, n = 20 and 25, respectively. (d) Photomicrograph of representative sections from spleens of WTnTg, WTTg, and KOTg mice stained with haematoxylin and eosin (H&E) (40X magnification); and immunohistochemistry stains with anti-BCL-6 and anti-CD138 or anti-IRF4 antibodies (100X magnification). Scale bars are 100 µm. (e) The percentage of Ki-67+ CD19+ lymphoma cells in WTTg (n = 4) and KOTg (n = 6) spleens as determined by flow cytometry. (f) Number of Cleaved Caspase-3 (CC3)+ lymphoma cells derived from WTTg and KOTg spleens as determined by immunohistochemistry, n = 3 and 4, respectively. (g) Photomicrograph of representative sections from spleens of WTTg and KOTg mice immunohistochemistry stained with anti-CC3 antibody. For (c), (e) and (f), data show mean ± SD; student t-test was used for data analysis: * p < 0.05; ns: no significant difference.
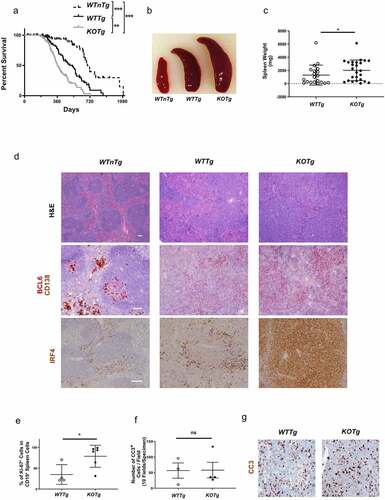
Figure 3. RhoH interacts with KAISO and deletion of Rhoh is associated with decreased nuclear content of KAISO in lymphoma cells. (a) Western blot analysis showing the expression of KAISO in the whole cell lysates (top panel), cytoplasmic (middle panel) and nuclear (lower panel) fractions of sorted CD19+ spleen cells isolated from WTTg and KOTg mice. (b) Intensity of KAISO Western blot bands by Image J analysis in the whole cell lysates, cytoplasmic, and nuclear fractions of sorted CD19+ spleen cells isolated from WTTg and KOTg mice. KAISO band intensity is normalized to the internal control, and those values are normalized to the WTTg KAISO internal control normalized values, n = 3. (c) Western blot analysis of immunoprecipitation of whole cell lysates from a malignant KOnTg B cell line transduced with a retroviral vector expressing HA-RhoH (HA-RhoH) using an anti-HA monoclonal antibody or IgG control followed by immunoblotting with anti-KAISO monoclonal antibody. (d) KAISO protein expression by Western blot analysis in the whole cell lysates (top panel), cytoplasmic (middle panel), and nuclear (lower panel) fractions of a KOTg lymphoma cell line transduced with empty vector (Control) or HA-RhoH (RhoH). (e) Intensity of KAISO Western blot bands by Image J analysis in the whole cell lysates, cytoplasmic, and nuclear fractions of a KOTg lymphoma cell line transduced with empty vector (Control) or HA-RhoH (RhoH). For (a), (b), (d) and (e), β-Actin (ACTB) was used as a loading control for whole cell lysates, α-TUBULIN (TUBA) and LAMIN B1 (LMNB) were used as loading controls for cytoplasmic and nuclear fractions, respectively. KAISO band intensity is normalized to these internal controls, and those values are normalized to the KOTg-Control KAISO internal control normalized values, n = 3. All of the Western blots were performed three times, and representative figures of those blots are shown. Data show mean ± SD, * p < 0.05; ** p < 0.01; *** p < 0.001; ns: no significant difference (f) Subcellular localization of KAISO detected by immunofluorescence staining in a KOTg lymphoma cell line transduced with empty vector (Control) or HA-RhoH (RhoH). Immunofluorescence shows staining with anti-KAISO antibody (red) and nuclear staining with Hoechst33342 (blue). The arrows denote individual cells stained with KAISO. Scale bars are 5 µm. The images were captured by Leica TCS SP8 (STED One) at 100X magnification.
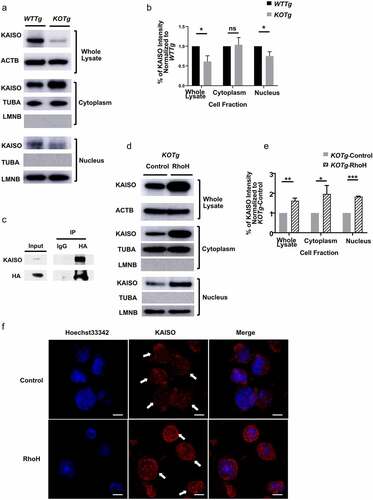
Figure 4. KAISO binds to the Bcl-6 promoter, and deletion of Rhoh leads to de-repression of the KAISO target Bcl-6 and the downregulation of the BCL-6 target Blimp-1. (a) Chromatin Immunoprecipitation (ChIP) was performed in a KOTg lymphoma cell line transduced with empty vector (Control) or HA-RhoH (RhoH) against anti-KAISO antibody or IgG control followed by qPCR with primers specific for known KAISO binding sites at the Bcl-6 promoter, including the two regulatory regions R1 (bp, −900~-450) and R3 (bp, +2165~+2423). The Flt3 gene promoter was selected as a negative control. n = 3; data show mean ± SD, * p < 0.05; ns: no significant difference. (b) BCL-6 expression by Western blot analysis in the whole cell lysates (top panel), cytoplasmic (middle panel), and nuclear (lower panel) fractions of sorted CD19+ spleen cells harvested from WTnTg, WTTg, and KOTg mice. (c) Intensity of BCL-6 Western blot bands by Image J analysis in the whole cell lysates, cytoplasmic, and nuclear fractions of sorted CD19+ spleen cells harvested from WTnTg, WTTg, and KOTg mice. BCL-6 band intensity is normalized to the internal control, and those values are normalized to the WTTg BCL-6 internal control normalized values, n = 4 for the whole lysates and n = 3 for the cytoplasmic and nuclear fractions. (d) BLIMP-1 expression by Western blot analysis in whole cell lysates of sorted CD19+ spleen cells isolated from WTnTg, WTTg, and KOTg mice shown. (e) Intensity of BLIMP-1 Western blot bands by Image J analysis in the whole cell lysates of sorted CD19+ spleen cells harvested from WTnTg, WTTg, and KOTg mice. BLIMP-1 band intensity is normalized to the internal control, and those values are normalized to the WTTg BLIMP-1 internal control normalized values, n = 4. α-TUBULIN (TUBA) and LAMIN B1 (LMNB) were used as loading controls for cytoplasmic and nuclear fractions, respectively, and β-Actin (ACTB) was used as a loading control for whole cell lysates. All of the Western blots were performed four times for the whole cell lysates and three times for the cytoplasmic and nuclear fractions. Representative figures of those blots are shown. Data show mean ± SD, * p < 0.05; *** p < 0.001; ns: no significant difference.
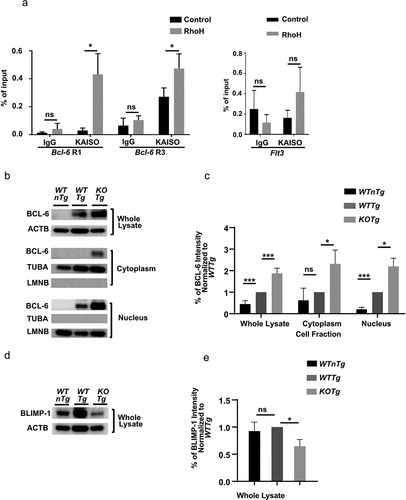
Figure 5. RhoH re-expression in lymphoma cells reverses alterations of BCL-6 and BLIMP-1 expression. (a) Western blot analysis showing the expression of BCL-6, HA tagged BCL-6, or HA tagged RhoH in the whole cell lysates of a KOTg lymphoma cell line transduced with empty vector (Control) or HA tagged RhoH (RhoH). (b) Intensity of total BCL-6 (BCL-6) and exogenous BCL-6 (HA tagged BCL-6) Western blot bands by Image J analysis in the whole cell lysates of a KOTg lymphoma cell line transduced with empty vector (Control) or HA tagged RhoH (RhoH). BCL-6 and HA tagged BCL-6 band intensity is normalized to the internal control, and those values are normalized to the KOTg-Control BCL-6 or HA tagged BCL-6 internal control normalized values, respectively, n = 3. (c) Western blot analysis showing the expression of BCL-6, HA tagged RhoH, and BLIMP-1 in the whole cell lysates (top panel), cytoplasmic (middle panel), and nuclear (lower panel) fractions of a KOnTg B cell line transduced with empty vector (Control) or HA tagged RhoH (RhoH). (d) Intensity of BCL-6 Western blot bands by Image J analysis in the whole cell lysates, cytoplasmic, and nuclear fractions of a KOnTg B cell line transduced with empty vector (Control) or HA tagged RhoH (RhoH). BCL-6 band intensity is normalized to the internal control, and those values are normalized to the KOnTg-Control BCL-6 internal control normalized values, n = 3. (e) Bcl-6 mRNA expression by qPCR in a KOnTg B cell line transduced with empty vector (Control) or RhoH (RhoH) (right panel). β-Actin (Actb) was used as an internal control, n = 6; mean ± SD, * p < 0.05.) (f) Intensity of BLIMP-1 Western blot bands by Image J analysis in the whole cell lysates, cytoplasmic, and nuclear fractions of a KOnTg B cell malignant cell line transduced with empty vector (Control) or HA tagged RhoH (RhoH). BLIMP-1 band intensity is normalized to the internal control, and those values are normalized to the KOnTg-Control BLIMP-1 internal control normalized values, n = 3. For (a) and (c), β-Actin (ACTB) was used as a loading control for whole cell lysates; for (c), α-TUBULIN (TUBA) and LAMIN B1 (LMNB) were additionally used as loading controls for cytoplasmic and nuclear fractions, respectively. All of the Western blots were performed three times, and representative figures of those blots are shown. Data show mean ± SD, * p < 0.05; ** p < 0.01; *** p < 0.001; ns: no significant difference.
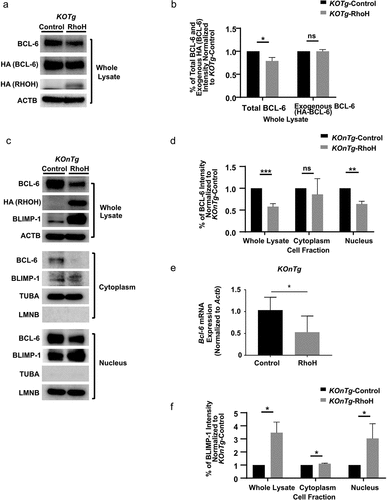
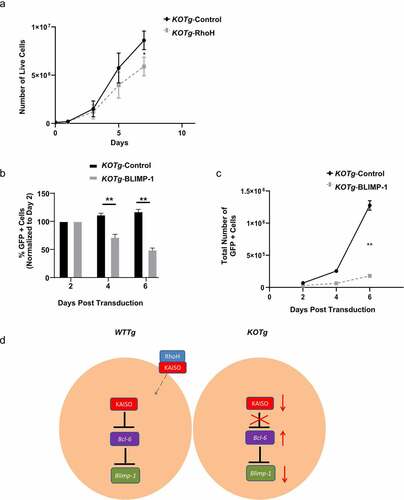
Figure 6. Re-expression of RhoH in KO lymphoma cells leads to decreased proliferation. (a) In vitro growth of a KOTg lymphoma cell line transduced with a RhoH-expressing retrovirus vector (KOTg-RhoH) versus empty vector (KOTg-Control), n = 4. (b) In vitro growth of a KOTg lymphoma cell line transduced with a BLIMP-1-expressing retrovirus vector with GFP (KOTg-BLIMP-1) versus empty vector with GFP (KOTg-Control). Flow cytometry was used to identify the GFP+ cell populations. The percentage of GFP+ cells on Days 4 and 6 were normalized to the percentage of GFP+ cells detected two days post transduction, n = 3. (c) In vitro growth of a KOTg lymphoma cell line transduced with a BLIMP-1-expressing retrovirus vector with GFP (KOTg-BLIMP-1) versus empty vector with GFP (KOTg-Control), n = 3. Flow cytometry was used to identify the GFP+ cell populations. Data show mean ± SD. * p < 0.05, ** p < 0.001. (d) Working model for RhoH involvement in tumour cell proliferation. In WTTg cells without RhoH deficiency, RhoH interacts with KAISO, and KAISO is present in the nucleus where it represses the transcription of Bcl-6. Transcription factor BCL-6 represses the Blimp-1 gene, which codes for the BLIMP-1 transcription factor important in the terminal differentiation of B cells into plasma cells. In KOTg cells with RhoH deficiency, decreased KAISO levels result in de-repression of Bcl-6. BCL-6 expression increases, which is followed by decreased BLIMP-1 expression.