Figures & data
Table 1 Frequency of NY-ESO-1 responding T cells from the peripheral blood of healthy donors (A) NY-ESO-1-specific T cells after 14 d priming
Table 1 (B) NY-ESO-1-specific T cells after GMP-grade isolation and expansion
Figure 1. GMP grade isolation and expansion of NY-ESO-1-specific T cells. (A and B) Peripheral blood mononuclear cells (PBMCs) from healthy donors were pre-sensitized with NY-ESO-1 overlapping peptides and IFNγ-secretion was analyzed after re-stimulation with NY-ESO-1 (as indicated below) followed by IFNγ-based enrichment and expansion using good manufacturing processes (GMP). (A) Time schedule of the protocol for generating NY-ESO-1-specific T cells using overlapping peptide pools of NY-ESO-1. (B) Frequencies of IFNγ+CD4+ and IFNγ+ CD8+ T cells directly before IFNγ enrichment and in the final T-cell product (after IFNγ enrichment and 14 d of expansion). Analyses were performed using overlapping peptide pools and determined by intracellular IFNγ staining after 6 h of re-stimulation with NY-ESO-1 antigen-pulsed or actin S control antigen (ACTS) dendritic cells (T cells: DC 10:1). Background values induced by stimulation with overlapping ACTS control peptide pools were subtracted. GMP grade large scale T-cell generation was done from 4 donors as proof of principle.
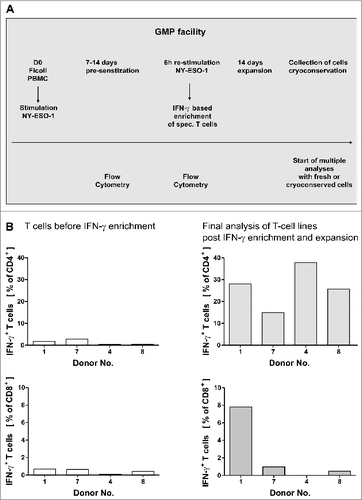
Figure 2. NY-ESO-1-specific proliferation, cytokine expression and CD107a expression of T cells with no alloreactive immune responses. (A) CD4+ and CD8+ T cells show a specific proliferation in response to NY-ESO-1. T cells of the final in vitro expanded T-cell products from 4 healthy donors were stained with carboxyfluorescein diacetate succinimidyl ester (CFSE) and stimulated with NY-ESO-1 or control actin peptide (ACTS) overlapping peptide pool-pulsed dendritic cells (DCs), respectively. After 6 d cells were re-stimulated for 6 h with the same antigens and analyzed by flow cytometry. Bars represent NY-ESO-1-specific proliferation and background proliferation (ACTS) for each donor ; representative CFSE staining combined with IFNγ analysis of T cells from donor 4. (B) T cells show a TH1 driven CD4 response to NY-ESO-1. CFSE-stained CD4+ T cells were analyzed for production of the cytokines IFNγ, TNFα and IL-10 by intracellular staining and flow cytometry (n = 4 donors). Results are mean +/− SD; representative staining of T cells from donor 1. (C) CD4+ T cells show cytolytic responses to NY-ESO-1. T-cell products of donors 1 and 4 were analyzed in 4 independent experiments for CD107a expression after 6 h of re-stimulation with NY-ESO-1 or ACTS overlapping peptide pools pulsed DCs. Bars show mean results of 4 experiments +/− SD and the results of different DC:T-cells ratios during re-stimulation are shown; representative staining of T cells from donor 4. (D) For final analysis of T-cell lines, T cells from donor 1, 7 and 4 were re-stimulated for 6 h with overlapping peptide (NY-ESO-1, ACTS) pool-pulsed dendritic cells (DCs) and analyzed by immunostaining and multispectral fluorescence cytometry. Bars represent mean values of double positive cells gated on CD4+/IFNγ+ T cells. T-cell subpopulations were defined as naïve T cells (CD27+/CD28+, CD62L+/CD45RO−), central memory T cells (CD62L+/CD45RO+, CD45RA−/CCR7+) and effector memory T cells (CD62L/CD45RO+, CD45RA−/CCR7− and CD27−CD28+).
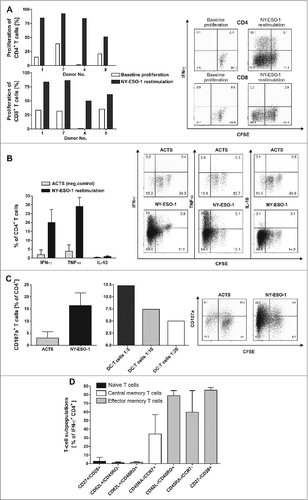
Figure 3. T-cell lines show effector memory phenotype with TH1 cytokine profile that do not include regulatory (T)cells. (A) There was no induction of alloreactivity when T cells of the final T-cell products and PBMCs of the same donor from donor 1, 7 and 4 were stimulated with allogeneic PBMCs and proliferation was analyzed via CFSE staining. Background proliferation using stimulation with autologous PBMCs was subtracted. (B) The final T-cell products were evaluated by immunostaining and flow cytometry for the frequency of regulatory T cells (Tregs) in comparison to peripheral blood mononuclear cells (PBMCs). T cells stimulated with 1000 U/mL IL-2 and CD3/CD28 beads were used as a positive control for appropriate gating of Tregs. Bars represent the mean results +/− SD of CD25+ FOXP3+ T cells from 3 different donors. (C–E) To quantify TH1 cytokines (C), chemokines (D) and growth factors (E) in the supernatants of T cells, the T-cell line from donor 1 was re-stimulated for 6 h with NY-ESO-1-or ACTS-pulsed DCs and supernatants were analyzed in a multiplex magnetic cytokine assay. All multiplex analyses represent mean results of 4 wells from 2 undiluted and 2 diluted (1:2, so values multiplied by 2) +/− SD of a T-cell line from donor 1.
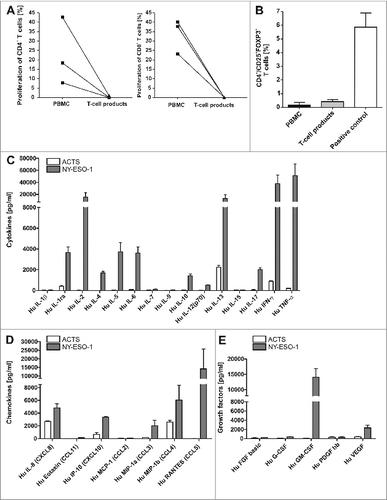
Figure 4. Recognition of a HLA-DR-binding peptide and naturally processed peptides. (A) As generation of NY-ESO-1-specific T cells was performed with an antigen independent of HLA types, we assessed MHC-binding specificity with a HLA Class II peptide predicted to bind HLA-DRB1*1501 according to SYFPEITHI database and synthesized this peptide (NY-ESO-185–99). NY-ESO-1-specific T cells from of donor 1 were re-stimulated with dendritic cells (DCs) pulsed with 5 µg/mL and 0.05 µg/mL NY-ESO-1 85–99 or control antigens (overlapping pools of NY-ESO-1, Class II matched background control filamin A peptide, positive control SEB) for 6 h. Specific responses were analyzed through expression of TNFα, IFNγ and IL-2. Recognition of the peptide is demonstrated by the bars depicting the mean values of 4 independent experiments +/− SD) (B) To confirm MHC Class II dependent recognition, HLA-DR, DB, DQ blocking antibodies were added to 3 of the T cell-stimulations described above. Bars show the specific blocking of T-cell peptide recognition using anti-HLA-DR, DB, DQ antibodies as mean values of 3 independent experiments +/− SD. (C) Immunostaining and cytofluorimetric analysis for IFNγ, TNFα and IL-2 to assay MHC Class II dependent peptide-specificity. (D) Recognition of naturally processed peptides: NY-ESO-1-specific T cells from donor 1 were re-stimulated with DCs in the presence of recombinant NY-ESO-1 protein or appropriate controls (negative = recombinant proteins Actin C and Survivin; positive control = SEB) for 16 h. Analysis for protein specificity represent 4 independent experiments +/− SD. Specific responses were analyzed through expression of TNFα, IFNγ and IL-2. (E) Blocking of the protein recognition by HLA-DR, DB, DQ antibodies: Bars demonstrate mean cytokine secretion of 3 independent experiments +/− SD. (F) Immunostaining and cytofluorimetric analysis for IFNγ, TNFα and IL-2 to assay MHC-Class II dependent peptide-specificity.
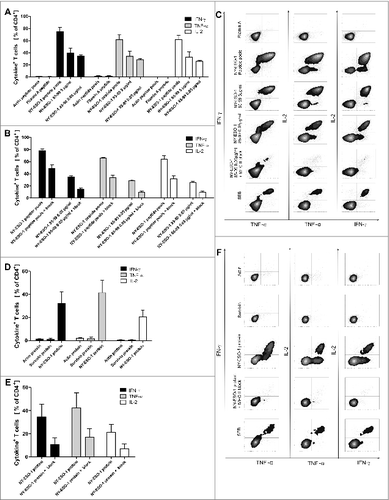
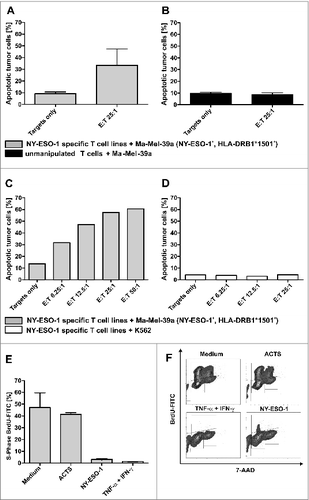
Figure 5. Antitumor responses induced by NY-ESO-1 specific T cells. (A) Cell-mediated antitumor response were assessed by co-culturing donor-derived T cell lines with melanoma cells. NY-ESO-1_specific T cells from donor 1 were re-stimulated for 4 h with the NY-ESO-1 expressing cell line Ma-MEL-39a, that was partially matched to the T-cell donor (HLA-DRB1*1501). Analysis of 4 independent experiments show an induction of apoptosis at an effector to target (E:T) cell ratio of 25:1. (B) Un-manipulated T cells from donor 1 did not induce apoptosis of Ma-Mel-39a cells, demonstrating the specific recognition of MaMel-39a by NY-ESO-1 cells. (C) Titration of T cells demonstrates a T-cell dependent increase of apoptosis in Ma-Mel-39a. (D) Absent induction of apoptosis in K562 cells by NY-ESO-1-specific T cells excludes non-specific toxic effects. (E) Supernatant-mediated anti-tumor response: NY-ESO-1-specific cell lines from donor 1 were re-stimulated with overlapping pools of peptides from NY-ESO-1 or Actin S (ACTS). Supernatants were collected 6 h later and co-incubated for 5 d with the primary melanoma cell line WM115 and BrdU incorporation was analyzed by flow cytometry. Controls were performed using medium alone and 100 ng/mL IFNγ + 20 ng/mL TNFα. DNA amount was analyzed by counterstaining with 7-AAD. Analyses of 2 independent experiments demonstrate a cell cycle arrest in the cytokine melanoma cell line WM115. (F) Representative result of the flow cytometry analysis after BrdU and 7-AAD staining.