Figures & data
Figure 1. IL-18 is secreted by cancer cells and HCMV-infected cells, and enhances IFNγ production by human Vδ2neg γδ T cells within PBMCs. (A) IL-18 or (B) IL-1β and IL-12 secretion by cancer cell lines. Cancer cell lines were cultured for 48 h and the secretion of cytokines was measured by ELISA from cell culture supernatants. Results are normalized by the same amount of cells used for each cell line. HUVEC endothelial cells were infected with HCMV at various multiplicities of infection (MOIs), and cell culture supernatant at 24 and 48 h post-infection was used to monitor (C) IL-18 or (D) IL-1β and IL-12 secretion by ELISA. Data are expressed as concentration of cytokines (pg/mL; mean ± SD; n = 3). (E) Example of Panδ immunotyping from whole blood. Peripheral blood mononuclear cells (PBMCs) were isolated from the blood of a kidney transplant patient and Panδ populations were quantified using anti-panδ and anti-Vδ2 antibodies within the CD3+ population by flow cytometry. (F) PBMCs isolated from a patient with expanded Vδ2neg population (>12% of CD3+) were incubated with various concentrations of anti-Vδ1 and anti-Vδ3 antibodies in the presence or absence of recombinant IL-18 (50 ng/mL) for 24 h at 37°C, then IFNγ secretion was measured by ELISA from cell culture supernatants (mean ± SD; n = 3). (G) Same as in (F) but PBMCs from another patient were treated with either anti-Vδ1 or anti-Vδ3 antibodies at various concentrations in the presence or absence of recombinant IL-18 or IL-1β (50 ng/mL) for 24 h at 37°C; then, IFNγ secretion was measured by ELISA from cell culture supernatants (mean ± SD; n = 3). (H) IFNγ secretion obtained from several kidney transplant patient's PBMCs was normalized by the same number of Vδ2neg γδ T cells and plotted as raw data. Anti-Vδ indicates treatment with combined anti-Vδ1+anti-Vδ3 antibodies (10 μg/mL each). (I) IL-18 response is represented as fold induction of IFNγ secretion after IL-18 treatment (anti-Vδ2neg +IL-18/anti-Vδ2neg). ★, P < 0.05.
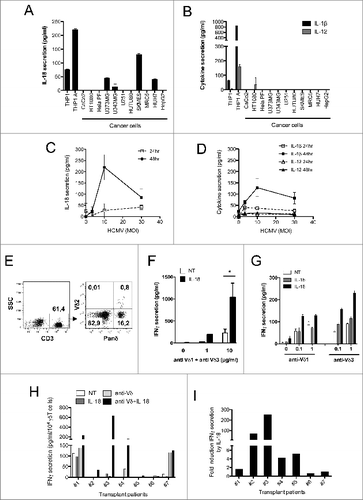
Figure 2. For figure legend, see next page. Figure 2 (see previous page). IL-18 alone enhances IFNγ production by purified Vδ2neg γδ T cells in a TCR-dependent manner. Human Vδ2neg γδ T-cell clones including (A) Vγ4Vδ5 or (B) Vγ8Vδ3 purified from human PBMCs were cultured through polyclonal activation and incubated with various concentrations of anti-CD3 in the presence or absence of IL-18 or IL-1β for 24 h at 37°C; then, IFNγ secretion was measured by ELISA from cell culture supernatants (mean ± SD; n = 3). The same procedure was applied to purified Vδ2neg γδ T polyclonal cell lines isolated from kidney transplant patients, including (C) Vδ1+Vδ3, or (D) Vδ1, or (E) Vδ5 γδ T cells. Polyclonal cell lines were incubated with various concentrations of γδ TCR agonists (anti-Vδ1, or anti-Vδ3, or anti-CD3 antibodies) in the presence or absence of either IL-18 or IL-1β for 24 h at 37°C. Anti-CD57 was used as negative control for TCR stimulation. IFNγ secretion was then measured by ELISA from cell culture supernatants (mean ± SD; n = 3). (F) Vδ2 γδ T polyclonal cell lines were isolated from PBMCs of 2 healthy donors and cultured through polyclonal activation using 4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMBPP) and IL-2, then incubated with various concentrations of γδ TCR agonist anti-Vδ2 in the presence or absence of either IL-18 or IL-1β for 24 h at 37°C. IFNγ secretion was then measured by ELISA from cell culture supernatants (mean ± SD; n = 3). (G) A Vδ1 γδ T polyclonal cell line was treated with anti-CD3 in the presence or absence of various cytokines alone or in combination for 24 h at 37°C. IFNγ secretion was then measured by ELISA from cell culture supernatants (mean ± SD; n = 3). (H) A Vδ1 γδ T polyclonal cell line treated with anti-CD3 and cell culture supernatant was used to monitor IFNγ, IL-18, or IL-12 secretion by ELISA. LPS/ATP-treated THP-1 cells were used as positive control for cytokine secretion (mean ± SD; n = 3). (I) The various Vδ2neg γδ T-cell clone and polyclonal cell line responses to IL-18 are represented as fold induction of IFNγ secretion by IL-18 (IL-18 + TCR agonist/TCR agonist).
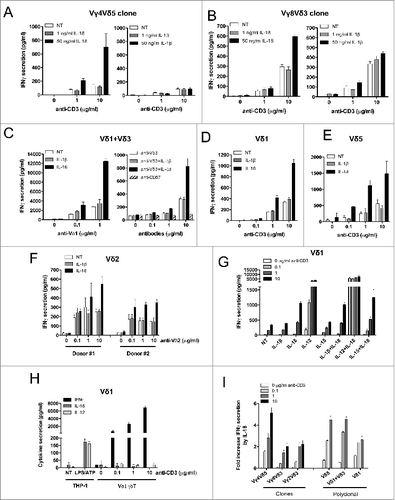
Figure 3. γδ TCR signaling increases membrane IL-18Rβ chain expression. (A) A Vδ1 γδ T polyclonal cell line, Vγ9Vδ1 cell clone, or B-cell lymphoma BEBV cell line were incubated with various concentrations of anti-CD3 for 24 h at 37°C, and immunostained with PE-conjugated anti-IL-18Rβ or IgG-PE control (for the Vδ1 γδ T polyclonal cell line). The expression of membrane IL-18Rβ chain was determined by flow cytometry within the healthy cell population. IL-18Rβ-PE+ cells are shown in the gate with the percentage of positive cells as indicated. (B) Bar graph summarizing IL-18Rβ-PE+ cells (%) from various Vδ2neg γδ T-cell clones (Vγ9Vδ1) and polyclonal cell lines (Vδ1, Vδ1+3, Vδ5) after incubation with various concentrations of anti-CD3. Data are representative of at least 3 independent experiments with similar results (mean of at least 5.103 cells).
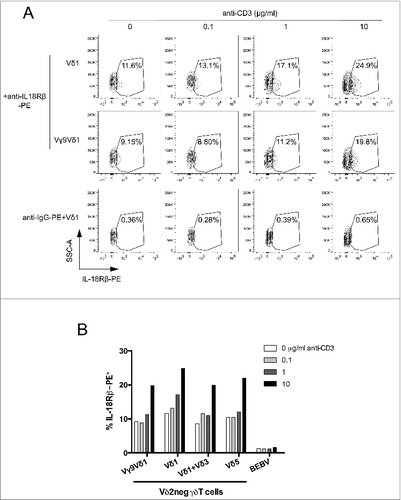
Figure 4. Soluble IL-18 secreted by cancer cells and HCMV-infected cells enhances IFNγ production by human Vδ2neg γδ T cells, in a TCR-dependent manner. (A) Conditioned culture supernatants of various cancer cell lines, or fresh media, were isolated after 48 h at 37°C, cleared by centrifugation, and incubated with a Vδ1 γδ T polyclonal cell line in the presence of various concentrations of coated anti-CD3 for 24 h at 37°C. IFNγ secretion was then measured by ELISA from cell culture supernatants (mean ± SD; n = 3). Results are normalized by the same amount of cells used for each cancer cell line. (B) Conditioned culture supernatants from various amounts of U373MG cancer cells, or fresh media, were isolated after 48 h at 37°C, cleared by centrifugation, and incubated with a Vδ1 γδ T polyclonal cell line in the presence of anti-CD3 for 24 h at 37°C. IFNγ secretion was then measured by ELISA from cell culture supernatants (mean ± SD; n = 3). (C) Conditioned culture supernatants of U373MG cancer cells, or HUVECs infected or not with HCMV (MOI 10), or fresh media, were isolated after 48 h at 37°C, cleared by centrifugation, and incubated with Vδ2neg γδT cells in the presence of anti-CD3 with or without anti-IL-18 or control anti-IL-10 antibodies (10 μg/mL). After 24 h at 37°C, IFNγ secretion was measured by ELISA from cell culture supernatants (mean ± SD; n = 3). As indicated, Vγ2Vδ3 T cell clone, Vδ1, and Vδ1+3 polyclonal cell lines were tested. ★, P < 0.05.
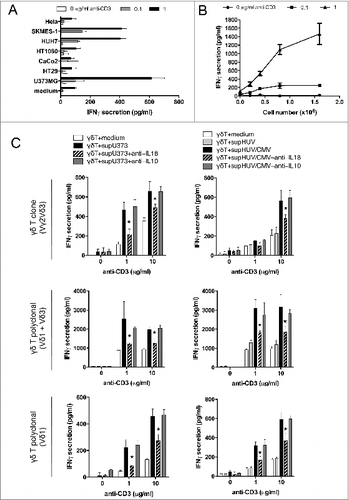
Figure 5. IL-18 produced by cancer cells and HCMV-infected cells contributes to IFNγ production by human Vδ2neg γδ T cells in co-culture. (A) U373MG cancer cells or (B) HUVECs uninfected or infected with HCMV (MOI 10) were cultured for 48 h at 37°C, and then co-cultured with a human Vγ4Vδ5 T-cell clone in the presence or absence of increased concentrations of anti-IL-18 or control anti-IL-10 antibodies. After 24 h at 37 °C, IFNγ secretion was measured by ELISA from cell culture supernatants (mean ± SD; n = 3). ★, P < 0.05.
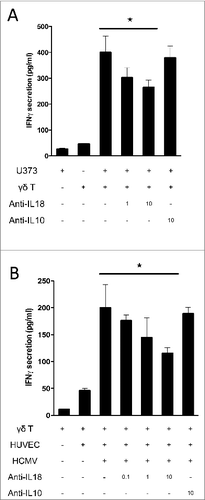
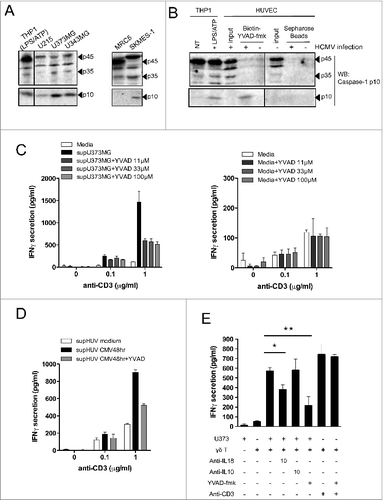
Figure 6. For figure legend, see next page. Figure 6 (see previous page). Active caspase-1 in cancer cells and HCMV-infected cells regulates the release of soluble molecules that enhance IFNγ production by human Vδ2neg γδ T cells. (A) Various cancer cell lines or non-transformed lung-derived MRC5 were cultured for 48 h, and lysed in denaturating buffer. Proteins were quantified to load the same amount of proteins per lane and then analyzed by SDS-PAGE immunoblotting (Western blotting) using anti-human caspase-1 p10 Ab. The p45 pro-caspase-1, p35, and p10 small catalytic subunit are indicated by dark arrowheads. LPS/ATP-treated THP-1 cells were used as the positive control for the presence of procaspase-1 p45 and p10. (B) HUVECs uninfected or infected with HCMV (MOI 10 for 48 h) were lysed in immunoprecipitation buffer (IP), and proteins were quantified to load the same amount of proteins per lane. Input indicates loading of cell lysates. THP-1 cells that were untreated or treated with LPS/ATP were used as positive control for the presence of procaspase-1 p45 and p10 using the anti-human caspase-1 p10 Ab. In parallel, solubilized caspase-1 p10 fragments contained in cleared supernatants after HUVEC cell lysis were precipitated by the addition of biotinyl-VAD-fmk; thereafter, biotinylated complexes were recovered by adding streptavidine-Sepharose beads, and the Sepharose-bound complexes were analyzed by Western blotting (WB) using anti-human caspase-1 p10 Ab. (C) U373MG cancer cells or (D) HUVECs that were uninfected or infected with HCMV (MOI 10) were cultured for 48 h at 37°C with or without various concentrations of Ac-YVAD-fmk (40 μmol/L for HUVECs). Conditioned culture supernatants (to the left) or fresh media (to the right) were then cleared by centrifugation and incubated with a Vδ1 γδ T polyclonal cell line for 24 h at 37°C, in the presence of anti-CD3. IFNγ secretion then was measured by ELISA from cell culture supernatants (mean ± SD; n = 3). (E) U373MG cancer cells were cultured for 48 h at 37°C with or without various concentrations of Ac-YVAD-fmk, and then co-cultured with a human Vγ4Vδ5 T-cell clone in the presence or absence of anti-IL-18, control anti-IL-10, or anti-CD3 antibodies. After 24 h at 37°C, IFNγ secretion was measured by ELISA from cell culture supernatants (mean ± SD; n = 3). ★, P < 0.05 and ★, P < 0.01.