Figures & data
Figure 1. DNA vaccine-induced anti-neu antibodies are successfully transferred from mothers to their pups and induce delayed mammary carcinoma onset in neu+ offspring. (A) Detection of vaccination-induced anti-neu antibodies in the milk and sera of control (white bars) and ECTM-(black bars) vaccinated mothers and in the sera of their 4-week-old offspring. **, p = 0.004; ***, P ≤ 0.0003, Student's t-test. Data are representative of 2 independent experiments and represented as mean ± SEM. (B) Detection of anti-neu IgG in control (white bars) and ECTM (black bars) offspring's sera collected from the first to the eighth week after birth. (C) Appearance of the first palpable mammary tumor in control (dotted black line, n = 12) and ECTM (continuous black line, n =26) neu+ offspring. Data are representative of 4 independent experiments. ***, P < 0.0001, Mantel–Haenszel Log-rank test. (D) ECTM offspring displayed a significant extension in overall survival as compared to control offspring. ***, p < 0.0003, Mantel-Haenszel Log-rank test.
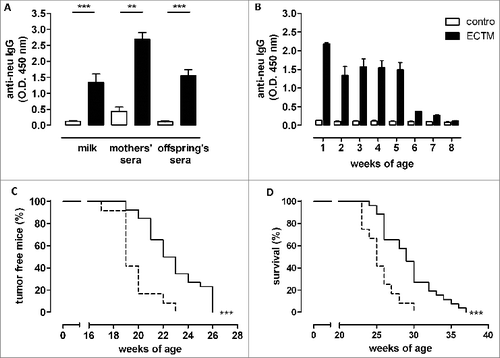
Table 1. Maternal immunization against neu hampers the growth of a transplantable mammary tumor
Figure 2. Maternal immunization against an oncoantigen, but not an unrelated antigen, delayed mammary carcinoma onset in neu+ offspring. (A) Detection of vaccination-induced anti-Amot antibodies in sera and milk of control (white) and pAmot (black) mothers and in the sera of their 4-week-old offspring. ***, P ≤ 0.0003, Student's t-test. (B) Tumor incidence in control (dotted black line, n = 11) and pAmot (continuous black line, n = 18) neu+ offspring. Data are representative of 3 independent experiments. **, p = 0.001, Mantel–Haenszel Log-rank test. (C) Western blotting analysis of β-galactosidase protein. Sera from control and LacZ mothers and their offspring were used as primary antibodies, recombinant β-galactosidase as positive control, and HSP90 protein as loading control. (D) Tumor incidence in control (dotted black line, n = 10) and LacZ (continuous black line, n = 8) neu+ offspring.
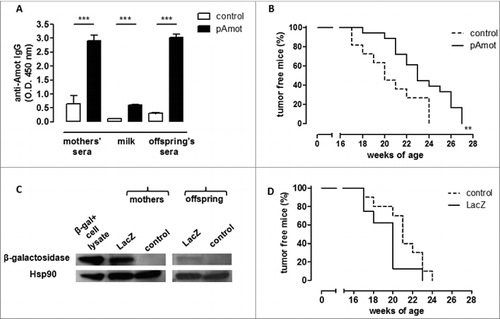
Figure 3. The presence of anti-neu antibodies and functional FcγRI/III are required to delay mammary carcinogenesis in neu+ offspring. (A) Tumor incidence of mammary carcinomas in neu+ offspring of BKO control (dotted black line; n = 7) and ECTM (continuous black line; n = 6) mothers. Data are representative of 2 independent experiments. (B) Characterization of IgG subclasses of anti-neu antibodies in the sera and in the milk of ECTM mothers and in the sera of their 3- to 4-week-old offspring. (C) Tumor incidence of mammary carcinomas in neu+ offspring of FcγKO control (dotted black line; n = 7) and ECTM (continuous black line; n = 5) mothers. Data are representative of 2 independent experiments.
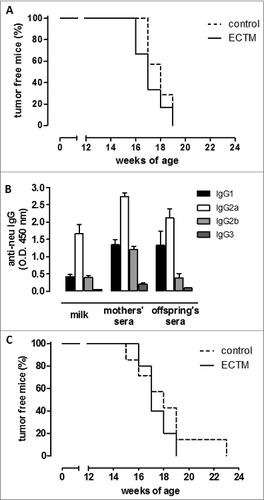
Figure 4. The passive transfer of maternal immunity induces an active humoral immune response in offspring. (A) Detection of EC–IgG immune complexes in mothers' milk and sera. (B) Detection of anti-neu IgM in mothers' milk and sera and in the sera of their 3- to 4-week-old offspring. (C) Presence of neu-specific IgM+ memory B cells in 5-week-old neu- and neu+ offspring. neu-specific IgM-secreting cells are expressed as SFU/1 ×106 B cells. In all panels, white bars refer to control vaccinated mice, black bars to ECTM vaccinated mice. Data show the mean ± SEM of values obtained from 2 to 3 independent experiments. *, p = 0.01; **, p = 0.004; ***, P ≤ 0.0007, Student's t-test.
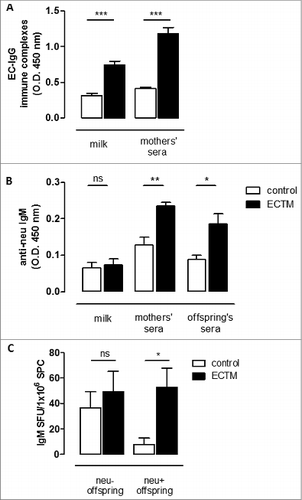
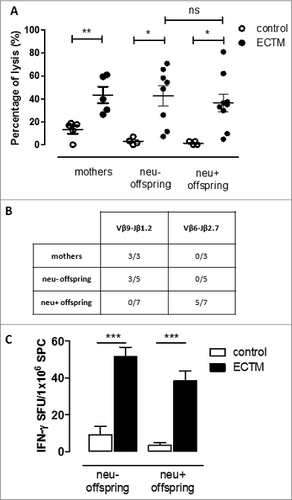
Figure 5. The passive transfer of maternal immunity induces an active cytotoxic immune response and the expansion of a distinct TCR repertoire in the offspring. (A) In vivo cytotoxic response against p63–71 peptide in control (white dots) or ECTM (black dots) mothers and in their neu- and neu+ 5-week-old offspring. (B) TCR repertoires in ECTM vaccinated mothers and in their neu- and neu+ offspring. Immunoscope analysis was conducted on cDNA pools obtained from LNs of ECTM mothers (n = 3) and their neu+ (n = 7) and neu- (n = 5) 5-week-old offspring. LNs cells were re-stimulated in vitro with the p63–71 peptide. Vβ9-Jβ1.2 and Vβ6-Jβ2.7 rearrangement frequencies are shown. Data are representative of 2 independent experiments. (C) T-cell response against p63–71 peptide quantified in vitro with an IFNγ-based ELISPOT assay. IFNγ-producing cells from control (white bars) and ECTM (black bars) neu- and neu+ offspring are expressed as SFU/1 ×106 SPC. *, p = 0.01; **, p = 0.005; ***, p = 0.0008, Student's t-test. Graphs display mean ± SEM and are representative of 2 independent experiments.