Figures & data
Figure 1. Expression of HLA-ABC in a panel of human breast cancer cell lines with and without overexpression of HER2. The indicated breast cancer cells were double-stained with APC-conjugated anti-HLA-ABC antibody and trastuzumab plus FITC-conjugated anti-human IgG antibody and subjected to flow cytometry analysis. (A). Flow cytometric data on HLA-ABC expression versus HER2 expression in each cell line in contour plots. (B). MFI values of HLA-ABC expression vs. MFI values of HER2 expression (left) and percentages of HLA-ABC-positive cells versus percentages of HER2-overexpressing cells (right) in scatter plots. Pearson correlation coefficients (r) and p values are shown. The numbers next to the dots in the scatter plots correspond to the numbers next to the names of breast cancer cell lines in A.
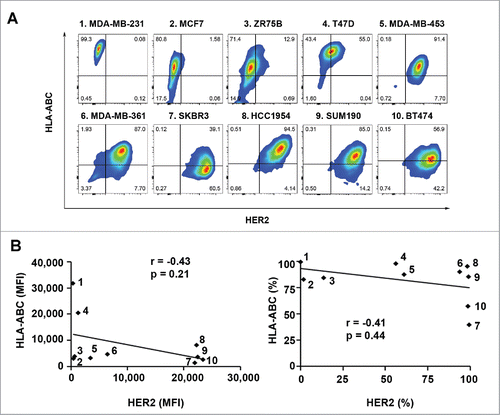
Figure 2. Effect of HER2 overexpression and knockdown on the level of HLA-ABC expression in breast cancer cells. (A). MCF7 and MCF7-HER18 cells were double-stained with APC-conjugated anti-HLA-ABC antibody and trastuzumab plus FITC-conjugated anti-human IgG antibody and subjected to flow cytometry analysis. Upper panel, flow cytometric data on HLA-ABC expression vs. HER2 expression in MCF7 and MCF7-HER18 cells in contour plots. Lower panel, MFI values of HLA-ABC and HER2 expression (left) and the percentages of cells expressing HLA-ABC and HER2 (right) in MCF7 and MCF7-HER18 cells. (B). The indicated HER2-overexpressing human breast cancer cells were transfected with one of two different HER2-specific siRNAs or control siRNA using Lipofectamine for 72 h in culture. The cells were double-stained for flow cytometry analysis of HLA-ABC and HER2 and the data were plotted as described in (A). * p < 0.05 compared with corresponding control.
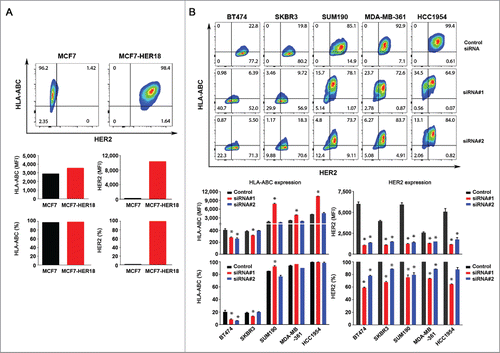
Figure 3. Effect of lapatinib on the level of HLA-ABC expression in HER2-overexpressing breast cancer cells. The indicated HER2-overexpressing human breast cancer cells were treated with vehicle (DMSO) or lapatinib (0.5 or 1.0 µM) for 24 h. After treatment, aliquots of cells were lysed for Western blot analysis with the indicated antibodies (A) or stained with APC-conjugated anti-HLA-ABC antibody for 30 min and subjected to flow cytometry analysis (B). Any cells undergoing apoptosis following treatment (Annexin-V-positive cells) were gated out before analysis of the flow cytometric data. Shown in (B) are the MFI values of HLA-ABC expression (left) and the percentages of HLA-ABC-positive cells in the cell lines treated with vehicle or lapatinib (right). * p < 0.05 compared with corresponding control.
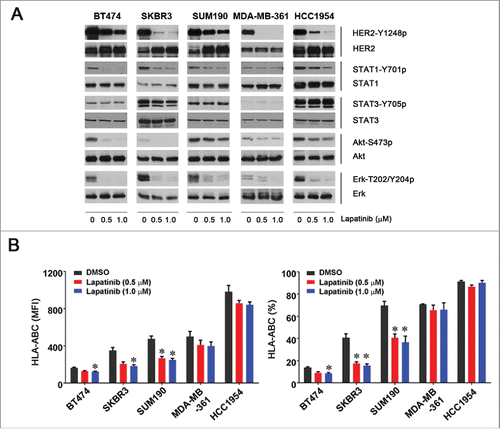
Figure 4. Increased HLA-ABC expression in HER2-overexpressing breast cancer cells by trastuzumab in the presence of PBMC. The indicated HER2-overexpressing human breast cancer cells were cultured in medium supplemented with 10% fetal bovine serum and were treated with 5 µg/mL (˜30 nM) trastuzumab or a control humanized IgG (h-IgG; bevacizumab) in mono-culture or co-culture with human PBMC at a ratio of 1:5 (cancer cells versus PBMC) for 48 h. After treatment, any PBMC grown in suspension were removed by washing the cells with PBS. The cells were then stained with APC-conjugated anti-HLA-ABC antibody and subjected to flow cytometry analysis. Any residual PBMC in the co-culture were gated out during flow cytometric analysis on the basis of the size difference between PBMC and the co-cultured breast cancer cells. Shown are the MFI values of HLA-ABC expression (upper) and the percentages of HLA-ABC-positive cells (lower). The data presented are from multiple experiments using PBMC from different donors. * p < 0.05 compared with corresponding control.
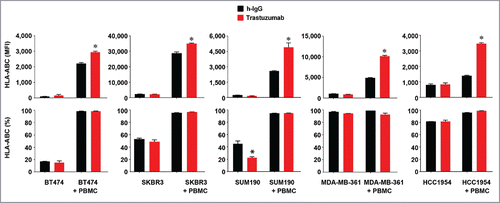
Figure 5. Increased HLA-ABC expression in HER2-overexpressing breast cancer cells by trastuzumab through engagement of NK cells and stimulation of IFNγ secretion. (A). BT474, SUM190, and HCC1954 cells were cultured in medium supplemented with 10% fetal bovine serum and were treated with 5 µg/mL (˜30 nM) trastuzumab or a control humanized IgG (h-IgG; bevacizumab) in mono-culture or co-culture with human 1.5×106 PBMC (at a ratio of 1:5, cancer cells vs. PBMC) for 48 h. Cell-free conditioned medium from each culture was used for detecting the presence of human IFNγ with an ELISA kit. * p < 0.05 compared with corresponding control. (B). BT474 and SUM190 cells were treated with the conditioned medium from the monoculture and co-culture described in (A) or with the conditioned medium plus an IFNγ-neutralizing antibody as indicated for 48 h. The cells were then stained with APC-conjugated anti-HLA-ABC antibody and subjected to flow cytometry analysis. Shown are the MFI values of HLA-ABC expression (upper) and the percentages of HLA-ABC-positive cells (lower). * p < 0.05 compared with corresponding control or compared with the group treated with an IFNγ-neutralizing antibody. (C). BT474 and SUM190 cells were cultured in medium supplemented with 10% fetal bovine serum and were treated with 5 µg/mL (˜30 nM) trastuzumab or a control humanized IgG (h-IgG; bevacizumab) for 24 h in co-culture at a ratio of 1:5 (cancer cells versus immune effector cells) with 1.5×106 NK cells (CD56+), monocytes (CD14+), or T cells (CD3+). The immune effector cells were sorted by flow cytometry from PBMC after staining of PBMC with PE-Cy7-conjugated anti-CD56 antibody, APC-conjugated anti-CD14 antibody, and FITC-conjugated anti-CD3 antibody. Conditioned medium from each culture was used for detecting the presence of human IFNγ with an ELISA kit. * p < 0.05 compared with corresponding control.
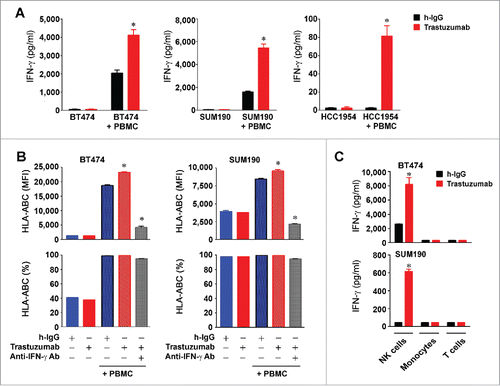
Figure 6. Increased expression of CD86 in HER2-overexpressing breast cancer cells by trastuzumab in the presence of PBMC. (A). HEK293, BT474, and SUM190 cells were stained with PE-conjugated anti-human CD80 antibody and PE-Cy7-conjugated anti-human CD86 antibody and subjected to flow cytometry analysis. (B). BT474 and SUM190 cells were cultured in medium supplemented with 10% fetal bovine serum and were treated with 5 µg/mL trastuzumab (˜30 nM) or a control humanized IgG (h-IgG; bevacizumab) in mono-culture or co-culture with human PBMC at a ratio of 1:5 (cancer cells vs. PBMC) for 48 h. The cells were then stained with PE-Cy7-conjugated anti-human CD86 antibody and subjected to flow cytometry analysis. Shown are the MFI values of CD86 expression (upper) and the percentages of CD86-positive cells (lower). * p < 0.05 compared with corresponding control.
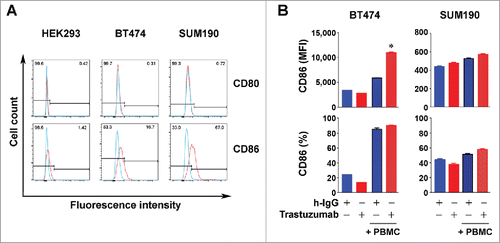
Figure 7. Increased expression of MHC-I and CD80/CD86 molecules by trastuzumab in HER2-overexpressing breast cancer cells in vivo. (A). 4T1 mouse mammary tumor cells transduced to overexpress HER2 (4T1/HER2) and SUM190 human breast cancer cells were cultured in medium supplemented with 10% fetal bovine serum in the presence or absence of mouse IFNγ (300 units/mL) or human IFNγ (300 units/mL) for 24 h. The cells were then stained with APC-conjugated anti-H-2Kd (for 4T1/HER2) or APC-conjugated anti-HLA-ABC antibody (for SUM190) and subjected to flow cytometry analysis. Shown are the MFI values of MHC-I expression (upper) and the percentages of MHC-I-positive cells (lower). (B). 4T1/HER2 cells (2×106 cells) were implanted in the mammary fat pad of female Swiss nude mice (4–6 weeks old). Two weeks after the tumors were palpable, the mice were treated once with 200 µg of trastuzumab or a control humanized IgG (rituximab) intraperitoneally. At 48 h after treatment, the mice were euthanized, the tumors were removed, and single cell suspensions were prepared and double-stained with APC-conjugated anti-H-2Kd antibody and trastuzumab plus FITC-conjugated anti-human IgG antibody. Cells were then subjected to flow cytometry analysis. Shown are the MFI values of H-2Kd expression (upper) and the percentages of H-2Kd positive cells (lower) gated for HER2-positive cells. *p < 0.05 compared with corresponding control. (C). Single cell suspensions from 4T1/HER2 tumors described in (B) were triple-stained with APC-conjugated anti-mouse CD80 antibody, PE-conjugated anti-mouse CD86 antibody and trastuzumab plus FITC-conjugated anti-human IgG antibody, and subjected to flow cytometry analysis as described in (A). Shown are the MFI values of CD80 and CD86 expression (upper) and the percentages of CD80- and CD86-positive cells (lower) gated for HER2-positive cells. *p < 0.05 compared with corresponding control.
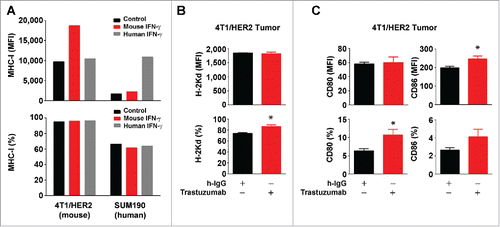
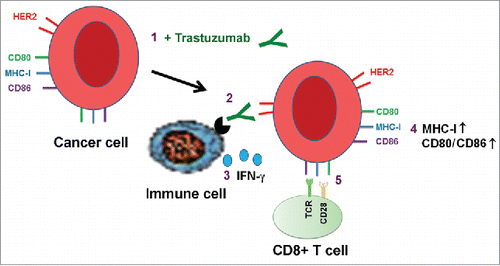
Figure 8. Proposed model of trastuzumab-induced upregulation of expression of HLA-ABC and CD80/CD86 T cell costimulatory molecules in cancer cells. Binding of trastuzumab to HER2 on breast cancer cell surface engages immune cells through interaction with the antibody's Fc fragment, leading to secretion of IFNγ, which upregulates the expression of MHC-I and CD80/CD86 molecules on targeted cells. The numbers show the sequence of events in the proposed model.