Figures & data
Figure 1. Schematic of PeptiCRAd (Peptide-coated Conditionally Replicating Adenovirus). PeptiCRAd is a novel cancer vaccine platform that exploits the natural immunogenicity of adenoviruses. OAds act as adjuvants for exogenous MHC-I tumor epitopes that are loaded onto the viral capsid by electrostatic interactions. These peptides could be known MHC-I epitopes or patient-derived tumor epitopes. Therefore, PeptiCRAd retains all the properties of a conventional oncolytic adenovirus (direct tumor-killing ability and possibility to express immune-stimulating molecules), however, it has a superior ability to stimulate a tumor-specific immune response.
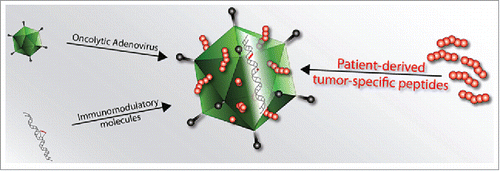
Figure 2. Physical characterization of the interaction between the modified MHC-I epitope SIINFEKL and OAd. (A) The net charge of SIINFEKL (dashed gray line, circles) or polyK-SIINFEKL (black line, triangles) is shown as a function of pH. (B) SPR was used to study the interaction between Ad5D24 oncolytic virus and increasing concentrations (0.15, 0.3, 0.6, 1.2, 2.4, 7.2, and 21.6 µM) of either SIINFEKL (dashed line) or polyK-SIINFEKL (solid line). (C) zeta potential (dashed gray line, left axis) and hydrodynamic diameter (solid black line, right axis) of virus-peptide complexes. Time-dependent study of complex´s zeta potential (D) and hydrodynamic diameter (E) after incubation at room temperature. Representative results from two different experiments are shown. The data are plotted as the mean ± SD (n = 3).
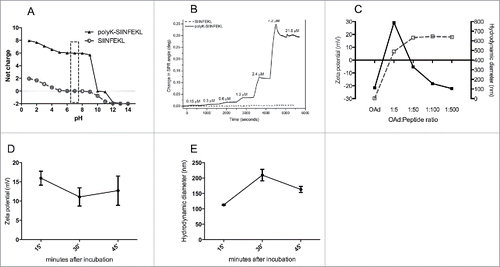
Figure 3. Cross-presentation of modified SIINFEKL analogs on MHC-I adsorbed or not adsorbed onto the viral capsid. (A) C57BL/6 fresh spleenocytes were incubated with SIINFEKL, the amino caproic acid-containing SIINFEKL-AHX-polyK, the C-terminus-extended SIINFEKL-polyK or the N-terminus-extended polyK-SIINFEKL. Cross presentation was determined with APC anti-H-2Kb bound to SIINFEKL or isotype control antibodies. (B) Similar to (A), spleenocytes were infected with of OVA-PeptiCRAd, or incubated with peptides SIINFEKL or polyK-SIINFEKL. The data are shown as the mean ± SD (n=2). Significance was assessed using the unpaired student´s t-test; ** p < 0.01, *** p < 0.001.
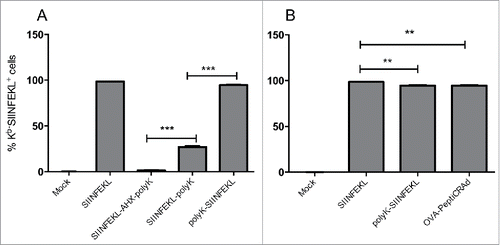
Figure 4. PeptiCRAd retains intact oncolytic activity and displays increased infectivity in cell lines with low CAR expression. (A) cell viability assay in different cell lines. The data are shown as the mean ± SD (n = 3). (B) Infectivity assay by ICC. Cells have been infected with 10 vp/cell of either naked OAd or OVA-PeptiCRAd. The average number of spots per visual field is presented (5 non-overlapping visual fields have been acquired and used for the generation of the means). Representative data from two independent experiments are shown as the mean ± SD (n = 2). Significance was assessed using the unpaired t-test with Welch's correction; * p < 0.05.
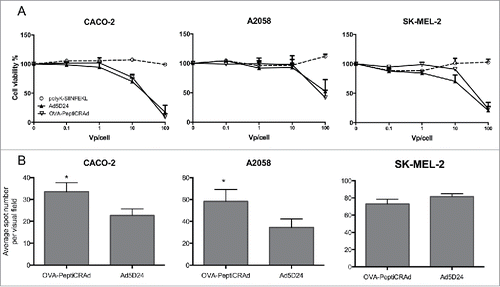
Figure 5. Antitumor efficacy of PeptiCRAd and immunological analysis of antigen-specific CD8+ T cells and DCs. C57BL/6 mice (n=8–9) received 3×105 B16-OVA cells in both flanks. Treatment was initiated nine days later and included saline solution (mock), peptide alone (SIINFEKL), virus alone (Ad5D24-CpG), a mixture of virus and SIINFEKL peptide (Ad5D24-CpG+SIINFEKL), and Ad5D24-polyK-SIINFEKL complex (OVA-PeptiCRAd). Mice were treated three times (on days 0, 2, and 7; black arrows). At day 7, before the third injection, mice from each group were sacrificed for early immunological analysis (n = 2–3). The late immunological analysis was performed on samples collected at the end of the experiment (tumors and spleens n = 3–4; lymph nodes n = 2–3). (A) Average tumor volume is represented excluding mice sacrificed at day 7. The percentage of SIINFEKL-Pentamer+ cells among CD19−CD8+ T-cells is reported for spleens (B), tumors (C) and draining lymph nodes (D) at early (left panels) and late (right panels) time points. Samples from Ad5D24-CpG group were collected at day 12. Data are presented as the mean ± SD. (E) The average tumor size at the end of the experiment was plotted against the average percentage of SIINFEKL-Pentamer+ CD8+ T cells at late time point. A correlation analysis was performed using a non-linear exponential model and the R square value is reported for each set of data. (E) Dendritic cells (CD19−CD3−CD11c+) showing a mature profile (CD86high) and cross-presenting SIINFEKL on their H2-Kb was determined in the spleens 7 and 16 d after the start of the treatment. The fold change between the two time points is presented. Statistical analysis was done using unpaired Mann–Whitney test; *p < 0.05, **p < 0.01.
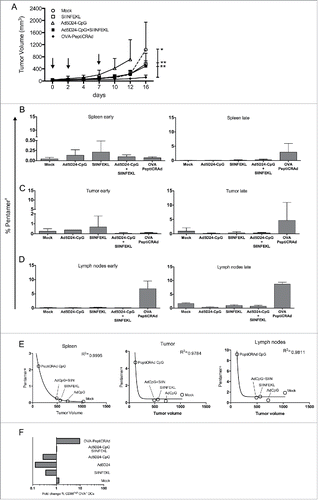
Figure 6. Targeting two tumor antigens with PeptiCRAd reduces the growth of both treated and untreated tumors. (A) C57BL/6 mice received 1×105 B16-F10 melanoma cells on the right flank and 3×105 B16-F10 cells on their left flank two days after the last treatment. Only the right tumor was treated. (B) The growth of the primary (right) tumor is presented as the mean ± SD (n = 6–7). (C) The size of the secondary (left) tumors at the end of the experiment is reported for each individual mouse. Tumor size oh hgp100-PeptiCRAd group is referred to day 11 before euthanasia of the group of mice. (D) The percentages of TRP-2- and hgp100-specific CD8+ T cells in spleens and inguinal lymph nodes form each mouse were normalized against mock, stacked into single columns and presented as the cumulative relative response for each experimental group. Samples from hgp100-PeptiCRAd group were collected at day 11. Significance was assessed by using the unpaired Mann–Whitney test; *p < 0.05; **p < 0.01.
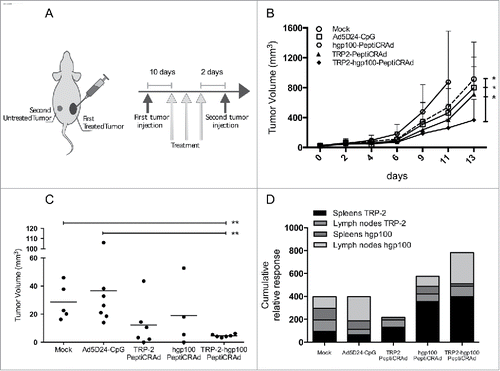
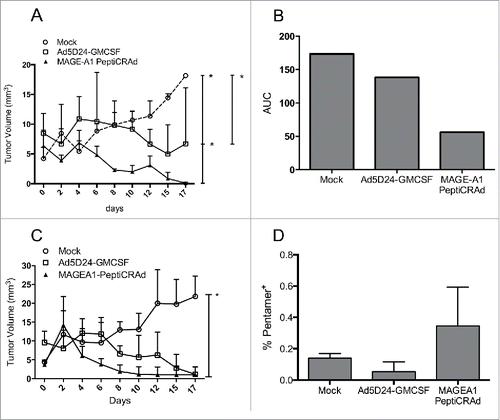
Figure 7. Efficacy of PeptiCRAd in humanized mice bearing human melanomas. Humanized and non-humanized mice bearing SK-MEL-2 human melanomas were treated with one of the following: (i) saline solution (mock), (ii) Ad5D24-GM-CSF, and (iii) MAGE-A1 PeptiCRAd. The tumor volume of the humanized mice (A) is presented as the mean ± SD (n = 3). (B) The area under the curve (AUC) relative to the size of the tumors of humanized-mice is presented. (C) The tumor volume of non-humanized mice (n = 2) is reported as the mean ± SD. (D) Percentage of MAGE-A1-specific CD8+ T cell population is presented as the mean ± SD (n = 2). Mann-Whitney test; *p < 0.05.