Figures & data
Table 1. Patients characteristics, clinical outcome and Melan-A-specific T-cell clones.
Figure 1. Melan-A-specific CD8+ T cells exhibit an enhanced polyfunctional profile after chemoimmunotherapy. (A) Representative dot plot gating strategy of multicolor intracellular staining for TNF-α (TNF), IFNγ (IFN), and Granzyme-B (GrB) in Melan-A-specific CD8+ T cells stimulated with HLA-A2+ Melan-A-expressing (Mel+) melanoma cell line (top panel). The multicolor histograms in the bottom panel show the quantification of all possible combinations of molecule co-expression, evaluated in the absence of stimulus (−) or after 4 h stimulation with A2+/Melan-A+ (Mel+) or A2+/Melan-A− (Mel-) melanoma cell lines. (B–C) Pooled results from all T-cell clones evaluated (n = 14 from two patients of Arm1, n = 27 from three patients of Arm2), isolated before (PRE) and after therapy (POST), analyzed as percentage of positive cells (%, left) and integrated MFI (iMFI, right) (see Material and Methods for details). Data are displayed singularly (B) and simultaneously (C). Each dot represents the mean value from two to four independent experiments performed with a single T-cell clone. The mean ± SEM of each indicated sample group is shown. Polyfunctionality profile (TNF-α+IFNγ+GrB+) is also shown as box-and-whisker diagram, with 5–95 percentile. */♦p ≤ 0.05, **/♦♦p ≤ 0.01, ***/♦♦♦p ≤ 0.001, ****/♦♦♦♦p ≤ 0.0001, Mann–Whitney two-sample test (*) and two-tail Student’s test (♦), respectively.
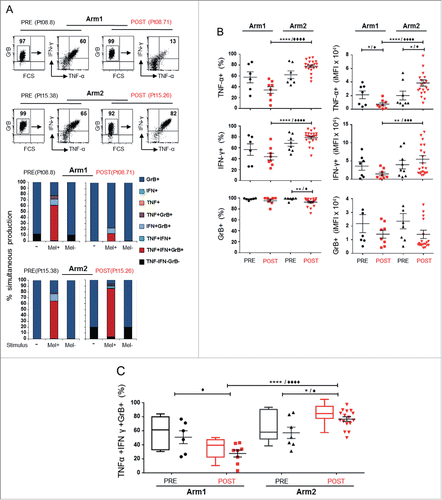
Figure 2. Melan-A-specific CD8+ T cells isolated after combined chemoimmunotherapy display pSer473-AKT expression unrelated to their differentiation stage. Analysis of AKT activation by Western blot in terms of phosphorylation of Ser473 (pSer473-AKT) and total AKT expression tested on whole cell extracts of 1.5 ×106 viable Melan-A specific clones (n = 26), isolated before (PRE) and after treatment (POST) with vaccination alone (Arm1) or DTIC plus vaccination (Arm2), 18 h following activation by plate-coated anti-CD3 mAb plus rIL-2. Gel loading control was performed analyzing β actin expression. The differentiation status (based on the expression of CD28 and/or CD27 co-stimulatory molecules, see ) for each clone is reported. Cells isolated from patients before the treatments evidence an expression of pSer473-AKT which relates with the differentiation status. Cell clones isolated after therapy (POST) are late differentiated (defined by the lack CD28 and CD27 molecules). Cells isolated from Arm1 (Arm1-POST) do not express pSer473-AKT according to their late differentiated profile, differently clones isolated from Arm2 patients (Arm2-POST) display a strong pSer473-AKT expression. Densitometric quantification (OD) of protein bands was determined by NIH ImageJ software and expressed p-AKT/AKT ratios are expressed either as numbers and columns.
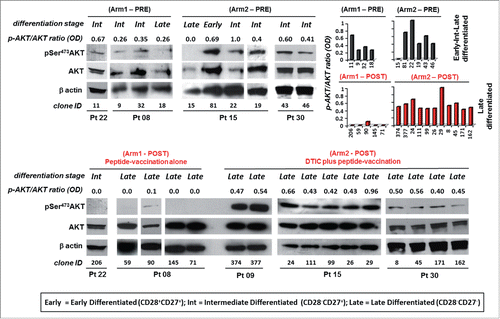
Figure 3. Melan-A-specific CD8+ T-cell clones isolated after DTIC plus vaccination display the highest level of PD-1 inhibitory receptor expression. (A) Representative flow cytometric analysis of multicolor staining, with the relative gating strategy, for simultaneous LAG-3 (L), TIM-3 (T) and PD-1 (P) expression. The multicolor histograms show the quantification of all possible combinations of molecule co-expression, for representative clones isolated either before (PRE) or at the end (POST) of both treatments. The staining was performed under basal cell culture conditions (−) or after over-night activation by plate-bound anti-CD3 mAb (2 µg/mL) (+). (B) Comparison from pooled data of inhibitory receptor profiles in PRE (black histograms) vs. POST (red histograms) treatment, displayed as single receptor expression, with (+) or without (−) anti-CD3 mAb activation. Each bar represents the mean percentage (± SD) of inhibitory receptor expression of Melan-A-specific T-cell clones isolated from patients of Arm1 (n = 2) and of Arm2 (n = 3), before (PRE, n = 6 for Arm1, n = 7 for Arm2) and after (POST, n = 7-8 for Arm1, n = 7–9 for Arm2) treatments. (C) The percentage of single or simultaneous inhibitory receptor expression in T-cell clones isolated at the end of both treatments (POST), with (■) or without (•) anti-CD3 activation. Each dot represents the mean value out of three–five independent experiments performed on a single T-cell clone. The mean ± SEM of each indicated sample group is shown. Black and green dots, clones negative or positive for pSer473-AKT, respectively, as assessed by Western blot analysis; gray dots, clones not tested for AKT phosphorylation. */♦p ≤ 0.05, **/♦♦p ≤ 0.01, ***/♦♦♦p ≤ 0.001, ****/♦♦♦♦p ≤ 0.0001, Mann–Whitney two-sample (*) test and two-tail Student’s test (♦), respectively.
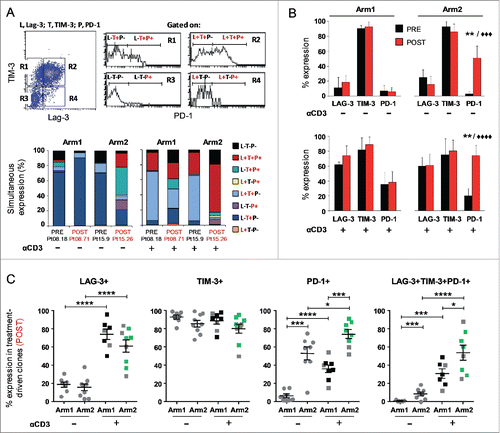
Figure 4. Proliferative potential of Melan-A-specific T-cell clones. Intracellular staining for Ki-67 expression of CTL clones (n = 12) isolated before (PRE) and after (POST) treatments from both Arms, performed 7 d after re-stimulation with allogenic irradiated PBMC, rIL-2 and PHA. Top panel: representative staining for each indicated sample group with anti-Ki-67 (open histograms) or isotype (filled histograms) mAbs, showing the presence of two positive cell populations with diverse amounts of Ki-67 molecule expression (low and high). Bottom panel: magnitude of low and high proliferating cells as measured by integrated mean fluorescence intensity (iMFI), (see Material and Methods for details). Results are shown as mean ± SD.
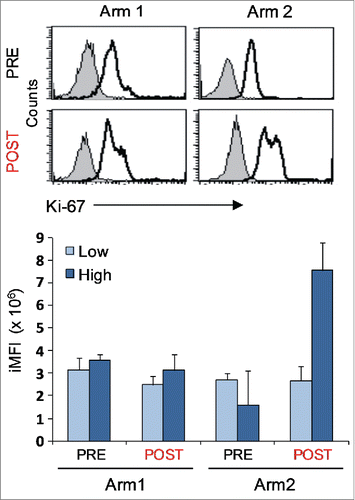
Figure 5. AKT-dependent antitumor activity of highly polyfunctional tumor-specific CD8+ T cells elicited by the combined chemoimmunotherapy. Modulation of TNF-α (TNF), IFNγ (IFN), and Granzyme-B (GrB) co-production by AKT or PD-1 blockade, in clones from Arm2 patient (Pt15). (A) Kinetic of IFNγ production after AKT pathway blockade, with a PI3K (Ly294002, PI3Ki) or AKT inhibitor molecule (MK-2206, AKTi). Figure shows the mean ± SD of three independent experiments performed on a representative Arm2 Melan-A-specific T cell clone (Pt15.29) after specific recognition of Melan-A-expressing cell line (Mel+). Results are shown as a percentage of IFNγ production (top panel) or as a percentage of inhibition with respect to the maximal baseline production (bottom panel). A specific Erk inhibitor (FR 180204) was used as internal control. (B–C) Flow cytometry analysis of multicolor staining from a representative Melan-A-specific clone (Pt15.29), showing the single % of production (B) or the co-production (C) of TNF-α, IFNγ, and GrB in the presence (1 h) or in the absence of inhibitors. TNF-α and IFNγ, but not GrB, are significantly reduced by the AKT blockade. PD-1 blockade, by specific anti-PD1 (α-PD-1) or anti-PD-L1 (α-PD-L1) mAbs (18 h) does not affect the TNF-α, IFNγ, and GrB production capability. (D) Pooled data from three different T-cell clones (Pt15.3, Pt15.26, Pt15.29 ) showing the mean ± SD of the % of inhibition evaluated under different blockade conditions, relative to baseline production.
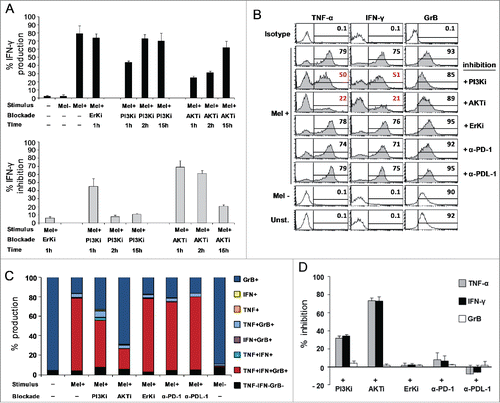
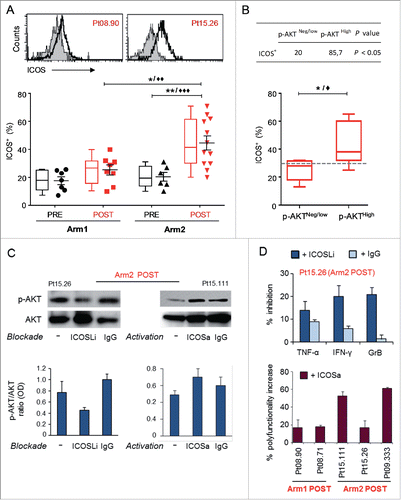
Figure 6. ICOS is upregulated in Melan-A-specific CD8+ T cells isolated after combined therapy and is involved in chemoimmunotherapy-induced AKT activation. (A) Top panel shows representative staining with anti-ICOS (open histogram) or isotype (filled histogram) mAbs, in two T-cell clones isolated from Arm1 (Pt08.90) and Arm2 (Pt15.26) patients after the treatments (POST). Bottom panel shows pooled data of ICOS expression in Melan-A-specific T-cell clones (n = 15 from two patients of Arm1, n = 18 from three patients of Arm2), isolated before (PRE) and after therapy (POST). Cells were stained after 48 h stimulation with plate-bound anti-CD3 mAb (2 µg/mL) plus rIL-2 and shown in both scatter-plot graph and box-and-whisker diagram, with 5–95 percentile. Each dot represents the mean value out of three–five independent experiments. The mean ± SEM of each group is shown. (B) Correlation between p-AKT and ICOS expression. T-cell clones isolated after both treatments were grouped according to pSerAKT expression levels and the percentage of ICOS+ cells (bottom panel) and shows higher expression of ICOS in AKThigh T cells. Top panel, percentage of clones with ICOS levels > 30% cut-off in p-AKTneg/low versus p-AKThigh groups. p value was calculated with one-tail Fisher’s exact test. */♦p ≤ 0.05, **/♦♦p ≤ 0.01, ***/♦♦♦p ≤ 0.001, Mann–Whitney two-sample test (*) and two-tail Student’s test (♦), respectively. (C) Left panel: effect of ICOS blockade on AKT activation (p-Ser473-AKT) tested in a representative Melan-A specific T-cell clone (Arm2, Pt15.26, POST), following activation by irradiated PBMC-derived APCs plus rIL-2 (−). Blockade of ICOS engagement with anti-ICOS-L (ICOSLi) mAb (10 µg/mL) induced a marked downregulation of p-AKT. Right panel: effect of ICOS engagement on AKT activation (p-Ser473-AKT) tested in a representative Melan-A specific T-cell clone (Arm2, Pt15.111, POST), following activation by anti-CD3 mAb (−). The anti-ICOS-agonist (ICOSa) mAb (12.5 µg/mL) induced upregulation of p-AKT. Gel loading control is represented by total AKT expression. Densitometric quantification of protein bands was determined by NIH ImageJ software. Columns show the mean ± SD of p-AKT/AKT ratios (OD) from two independent experiments. (D) Top panel: bars represent the mean % (± SD) of TNF-α, IFNγ and GrB inhibition by ICOS blockade from two experiments performed on Pt15.26 T cell clone (representative of three different T-cell clones tested). Bottom panel: bars represent the mean % (± SD) of the increased polyfunctional activity induced by ICOS co-stimulation, as calculated with respect to the isotype control mAb.