Figures & data
Figure 1. Tumors induce Wnt expression and DC-specific deletion of Wnt co-receptors LRP5 and LRP6 delays tumor growth in mice. Real-time PCR analysis for mRNA levels of different Wnts in CLNs (control lymph nodes ) from tumor-free mice or TDLNs (tumor-draining lymph nodes) on day 9 after MO4 (A), EL4 (B) and LLC (C) tumor inoculation in B6 WT mice (n = 5). Data are represented as fold increase over CLN (mean ± SEM). B16-OVA (D), EL4-OVA (E) and LLC (F) tumor progression in LRP5/6ΔDC and Control-FL mice (n = 6–8). Data are representative of three independent experiments and show mean values ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001.
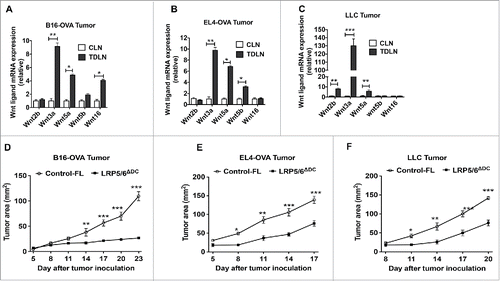
Figure 2. Wnt ligands in the TME activate the β-catenin/TCF pathway in DCs and DC-specific deletion of LRP5/6 enhances antitumor immunity in mice. (A–D) Representative dot plots and percentages of IFNγ+CD4+, IFNγ+CD8+, Granzyme B+CD8+, TNF-α+CD8+, IL-10+CD4+, Foxp3+CD4+ and IL-10+ CD8+ T cells isolated from MO4 tumors in Control-FL and LRP5/6ΔDC mice on day 14 post-inoculation (n = 6). (E) Cytokine concentrations in supernatants obtained after culture of TDLN lymphocytes with WT DCs loaded with B16 lysate for 48 h (n = 5). Data represents one of two experiments with similar results and shows mean values ±SEM. *p < 0.05; **p < 0.01; ***p < 0.001.
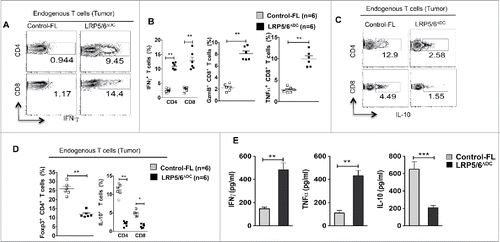
Figure 3. Loss of LRP5/6 in DCs promotes effector T cell differentiation whereas limits regulatory T cell differentiation. (A, B) In vivo OT-I CD8+ T cell differentiation in Control-FL and LRP5/6ΔDC mice in response to B16-OVA (MO4) tumors. Representative dot plots and percentages percentage of OVA-specific IFNγ+CD8+, Granzyme B+CD8+ and TNF-α+CD8+ T cells in tumors isolated from Control-FL and LRP5/6ΔDC mice (n = 6). (C, D) In vivo OT-II Treg differentiation in Control-FL and LRP5/6ΔDC mice in response to B16-OVA (MO4) tumors (n = 6). (E) MO4 melanoma and (F) EG7 tumor growth in LRP5/6ΔDC in response to α-CD4+ antibody or α-CD8+ antibody treatment (n = 6). Data represents cumulative percentage of OVA-specific Foxp3+CD4+ T cells in TDLNs and tumors isolated from Control-FL and LRP5/6ΔDC mice (n = 5). *p < 0.05; **p < 0.01; ***p < 0.001.
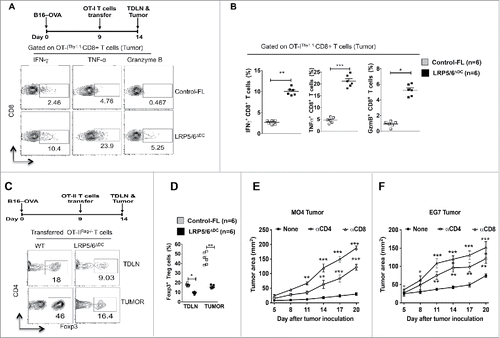
Figure 4. Loss of LRP5/6 in DCs differentially regulates pro- and anti-inflammatory molecules. (A–B) Quantitative real-time PCR analysis of Il-12p40, Il-12p35, Il-6, Il-12p19, Il10, Aldh1a1 and Aldh1a2 mRNA expression in CD11c+ DCs isolated from the TDLN of WTFL/FL and LRP5/6ΔDC mice on day 9 post-B16 tumor inoculation (n = 3). (C) Representative histograms of active β-catenin expression in CD11c+ cells from MO4 tumor TDLN of LRP5/6Δce and control-FL mice on day 9 after tumor inoculation. (D) Axin2 mRNA expression analyzed by qRT-PCR in TDLNs and CLNs of LRP5/6ΔAx and control-FL mice on day 9 after inoculation (n = 4). The result represents fold increase over the CLNs. (E) Representative histograms showing β-gal expression in CD11c+ cells from TCF/LEF-LacZ reporter mice after treatment with or without Wnt2b, Wnt3a, Wnt5a, Wnt5b, or Wnt16b (500 ng/mL) for 24 h in vitro. DCs were pooled from five to six mice, and the experiment was repeated at least two times. Data represents one of two experiments with similar results. *p < 0.05; **p < 0.01; ***p < 0.001.
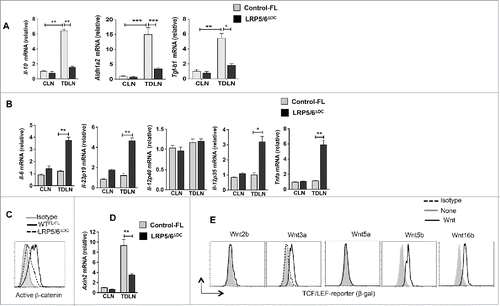
Figure 5. Loss of LRP5/6 in DCs promotes efficient capture of tumor-associated antigens and cross-priming of CD8+ T cells. (A, B) Control-FL and LRP5/6ΔDC mice were implanted s.c with B16-GFP tumor cells (n = 5). On day 7, TDLN and CLN were harvested and were tested using FACS for DC population. Data represents FACS plot and cumulative frequency showing percentage of CD11c+ GFP+ DCs (n = 8). (C–D) B16-OVA tumor-bearing Control-FL and LRP5/6ΔDC mice were adoptively transferred with CFSE-labeled naive OT-I T cells on day 9 (n = 6). On day 14, the TDLN was harvested and analyzed using FACS for OT-I T cell proliferation. Representative histograms showing cell division and percent proliferated OT-ICFSE cells. Data represents one of two experiments with similar results. ***p < 0.001. (E, F) B16-OVA tumor-bearing Control-FL and LRP5/6ΔDC mice were adoptively transferred with CFSE-labeled naive OT-II T cells on day 9 (n = 6). On day 14, the TDLN was harvested and analyzed using FACS for OT-II T cell proliferation. Representative histograms showing cell division and percent proliferated OT-IICFSE cells. (G) Representative FACS plot showing frequency of CD11c+ cells in the TDLN of B16-OVA tumor bearing Control-FL and LRP5/6ΔDC mice on day 14 after tumor inoculation. (H) Representative histogram shows the expression of MHC Class II by CD11c+ DCs in TDLN of TDLN of B16-OVA tumor-bearing Control-FL and LRP5/6ΔDC mice on day 14 after tumor inoculation.
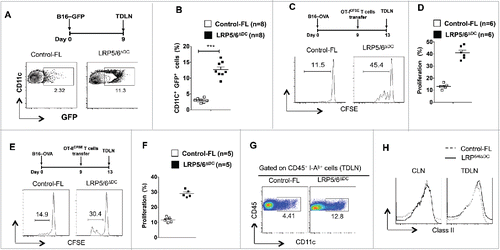
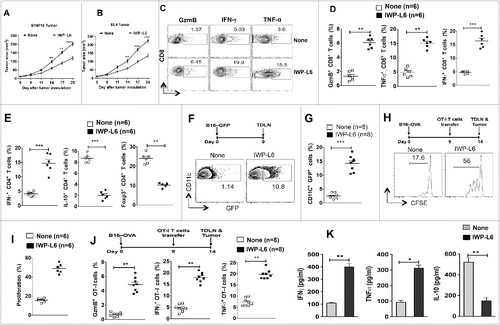
Figure 6. Pharmacological inhibition of Wnt signaling enhances antitumor immunity against established tumors. (A–B) B16F10 melanoma and EL4 tumor progression in WT mice treated with or without IWP-L6 (5 mg/kg) every 3 d from day 5 after tumor inoculation. Data are mean tumor size and are cumulative, representative of two independent experiments (n = 10–12 mice). (C–D) Representative dot plots and percentage of Granzyme B+CD8+, IFNγ+CD8+, and TNFα+CD8+ T cells isolated from MO4 melanoma on day 20 after inoculation (n = 6 mice). Mice were treated with or without IWP-L6 as described in A. (E) Representative percentage of Foxp3+CD4+, IFNγ+CD4+, and IL-10+CD4+ T cells isolated from MO4 tumors on day 20 after inoculation (n = 6 mice). Mice treated with or without IWP-L6 as described in A (F–G) Representative dot plots and percentage of GFP+CD11c+ DCs isolated from TDLNs on day 9 after B16-GFP tumor inoculation (n = 8 mice). Mice were treated with or without IWP-L6 on day 5 and 7. (H, I) MO4 bearing mice were adoptively transferred with CFSE-labeled naive OT-I T cells on day 9 (n = 6). Mice were treated with or without IWP-L6 on day 5, 8 and 11. On day 14, the TDLN was harvested and analyzed using FACS for OT-I T cell proliferation. Representative histograms showing cell division and percent proliferated OT-ICFSE cells. Data represents one of two experiments with similar results. (J) MO4-bearing mice were adoptively transferred with OT-I T cells from Rag−/− OT-I mice on day 9 after tumor inoculation. After 5 d, Granzyme B+CD8+, IFNγ+CD8+, and TNFα+CD8+ analysis was performed on CD45+Vα2+Vβ5.1/5.2+ T cells from TDLNs (n = 6–8 mice). Data are representative of two independent experiments and show mean values±SEM. (K) Cytokine concentrations in supernatants obtained after culture of TDLN lymphocytes with DCs loaded with B16 lysate for 48 h. Mice were treated with or without IWP-L6 as described in A (n = 5). Data are representative of two independent experiments and show mean values ± SEM. Statistical levels of significance were analyzed by the Student t test (unpaired). *p < 0.05; **p < 0.01; ***p < 0.001.