Figures & data
Figure 1. Combining intravenous delivery of live bacteria with adoptive T cell therapy prevents tumor relapse. (A) Diagram of the SIINFEKL-AAY repeat fused to EGFP. (B) MC57 parental, MC57-SIINFEKL high antigen-expressing cell line (MC57-SIINF-HI), and MC57-SIINFEKL low antigen-expressing cell line (MC57-SIINF-LO) were analyzed for SIINF expression by EGFP fluorescence using flow cytometry. (C) C57BL/6 mice were injected intravenously with CFSE-labeled OT-1 splenocytes. On the next day, A1-R SIINF or A1-R control bacteria were injected intravenously. Five days later, lymph nodes were harvested and proliferation of transferred OT-1 T cells was assessed by CFSE dilution using flow cytometry. (D) 2C mice were injected s.c. with MC57-SIINF-LO cells. After tumors were established for ≥ 2 weeks, mice were treated i.v. with monotherapeutic OT-1 splenocytes, A1-R control + OT-1, or A1-R SIINF + OT-1. Treatment with bacteria was 1 d prior to OT-1 T cell transfer. Each line represents an individual tumor-bearing mouse. Mice were compiled from four independent experiments, with three experiments containing all three treatment groups. Tumor relapse rate following combined treatment with OT-1 T cells and A1-R control was significantly lower (p < 0.05) compared to the relapse rate with OT-1 T cells alone. As a monotherapeutic control, 2C mice with long-established MC57 tumors were treated with live intravenous A1-R. Blue arrows represent the initial time of treatment. Green crosses represent mice that died during the experiment. (E) A relapsed MC57-SIINFEKL-LO tumor was re-isolated and analyzed for SIINFEKL expression by EGFP fluorescence. Data shown represent re-isolated tumors from two mice treated with OT-1 T cell monotherapy.
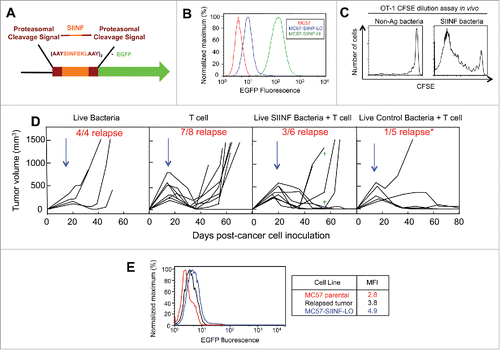
Figure 2. Combining intratumorally injected heat-killed bacteria with adoptive T cell therapy prevents tumor relapse. (A) MC57 SIY-LO cancer cells were injected s.c. onto the backs of OT-1 mice. Tumors were established ≥2 weeks prior to being treated with intravenously-injected adoptively-transferred activated 2C T cells, intratumoral HK bacteria, or combined 2C T cells + HK bacteria. Data represent mice compiled from three independent experiments, with each experiment consisting of each treatment group. The tumor relapse rate following combined treatment with HK bacteria and T cells was significantly lower (p < 0.05) compared to either monotherapy. Similarly, MC57 EGFP tumors were treated with the combination of HK bacteria and 2C T cells in two independent experiments. Blue arrows represent the initial time of treatment and the green cross represents one mouse death. (B) Re-isolated cancer cells from two relapsed MC57 SIY-LO tumors following monotherapy 2C T cell treatment were analyzed for SIY expression by EGFP fluorescence using flow cytometry. The MC57 parental and MC57 SIY-LO cell line, that was originally injected into mice, were used in this analysis. Data represent three relapsed tumors from two independent experiments.
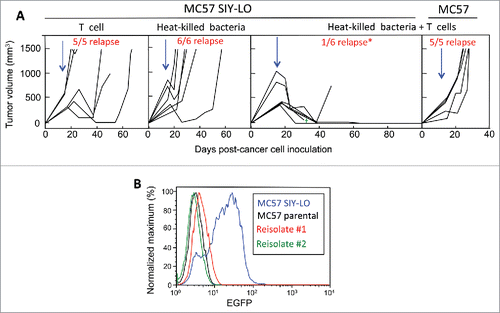
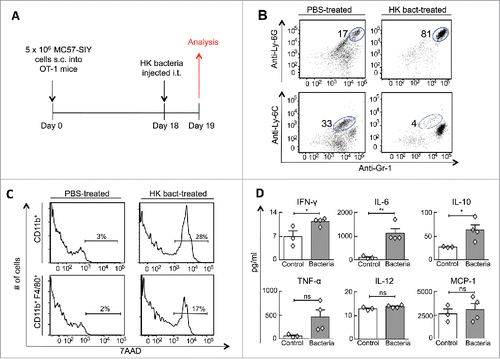
Figure 3. Intratumorally injected heat-killed bacteria transform the tumor microenvironment's cellular composition and cytokine profile. (A) MC57-SIY cancer cells were injected s.c. into OT-1 mice. Tumors were established for 18 d before receiving an intratumoral-injection with HK bacteria or PBS. One day later, mice were sacrificed and tumors were analyzed. (B) Tumor cell suspensions were analyzed for expression of Gr1, Ly6C, and Ly6G. The percentage of live CD11b+ cells identified as neutrophils (Gr1+ Ly6G+) and monocytes (Gr1+Ly6C+) are shown. Data are representative of four HK bacteria-treated mice and three control mice (untreated or PBS-treated) from two independent experiments. (C) Tumors from (B) were also analyzed for expression of CD11b, F4/80 and the 7AAD non-viability probe. 7AAD staining of gated myeloid cells (CD11b+) and gated macrophages (CD11b+, F4/80+) are shown. (D) Cytokine concentrations from tumor homogenates were quantified from tumors using a cytokine bead array. Data consist of four HK bacteria-treated mice and three control mice (untreated or PBS-treated) compiled from two independent experiments.