Figures & data
Figure 1. Characteristics of the breast cancer model used in this study. Breast cancers were induced in young (7-weeks-old) female BALB/c mice by implantation of medroxyprogesterone acetate (MPA)-releasing pellets followed by gavage with the DNA damaging agent 7,12-Dimethylbenz[a]anthracene (DMBA) for the following 6 weeks. The overall scheme of the experiment is shown in (A). Note that the interval between the last DMBA injection and the manifestation of palpable breast cancer lesions is rather variable. This interval is demonstrated for each mouse that was subsequently randomized for assignment to different treatment groups, as shown in (B). PBS, phosphate buffered saline; CsH, cyclosporine H; CTX, cyclophosphamide; MTX, mitoxantrone.
![Figure 1. Characteristics of the breast cancer model used in this study. Breast cancers were induced in young (7-weeks-old) female BALB/c mice by implantation of medroxyprogesterone acetate (MPA)-releasing pellets followed by gavage with the DNA damaging agent 7,12-Dimethylbenz[a]anthracene (DMBA) for the following 6 weeks. The overall scheme of the experiment is shown in (A). Note that the interval between the last DMBA injection and the manifestation of palpable breast cancer lesions is rather variable. This interval is demonstrated for each mouse that was subsequently randomized for assignment to different treatment groups, as shown in (B). PBS, phosphate buffered saline; CsH, cyclosporine H; CTX, cyclophosphamide; MTX, mitoxantrone.](/cms/asset/d23ee36f-2962-49ae-8122-f6d8b1a4c43d/koni_a_1139275_f0001_oc.gif)
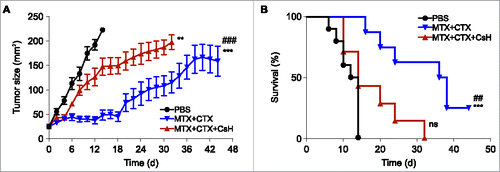
Figure 2. Effects of cyclosporin H on the efficacy of anticancer chemotherapy in a mouse model of breast cancers. (A) Immunocompetent BALB/c wild type (WT) mice bearing palpable hormone-induced mammary cancers received i.p. 5.17 mg/kg mitoxantrone (MTX) and 50 mg/kg cyclophosphamide (CTX) together with 30 mg/kg cyclosporin H (CsH) or an equivalent volume of phosphate buffered saline (PBS) and tumor growth was routinely assessed. Mice were treated when the tumor surface reached 25–35 mm2 (day 0) and tumor growth was routinely assessed starting from the apparition of each tumor. Results from one representative experiment out of two independent ones involving at least four mice/group and yielding similar results are illustrated. Data are represented as means ± SEM over time. **p < 0.01, ***p < 0.001 (Wald test), as compared to PBS-treated tumors; ###p < 0.05 (Wald test), as compared to MTX + CTX + CsH-treated tumors. Kaplan–Meier survival curves are shown in (B). n.s., non-significant, ***p < 0.001 (Log-Rank), as compared to PBS-treated tumors; ##p < 0.01 (Log-Rank), as compared to MTX + CTX+ CsH-treated tumors.