Figures & data
Figure 1. BTN3A triggering enhances TCR-mediated lysis of AML blasts by Vγ9Vδ2 T cells. AML blasts were pre-incubated for 1 h with Control Isotype, anti-BTN3A 20.1 agonist mAb (10 μg/mL) or O/N with Zoledronate (ZOL) (45 μM), before addition of effector cells. Each AML blast sample was tested with at least three different Vγ9Vδ2 T cells or NK cells donors. (A) Comparison of TCR agonists. Specific lysis of primary AML blasts (n = 25) by expanded Vγ9Vδ2 T cells from HV after treatment with BrHpp, ZOL, or anti-BTN3A 20.1 mAb. Data are shown for E:T 30:1. (B, C) Effect of TCR and BTN3A targeting. Primary AML blasts lysis (n = 11) by expanded Vγ9Vδ2 T cells from HV. (B) Vγ9Vδ2 T cells were pre-incubated for 30 min with anti-TCRγ9 mAb. The blasts were treated with ZOL or anti-BTN3A agonist 20.1 mAb. (C) The blasts were treated with ZOL, agonist (20.1) or antagonist (108.5) anti-BTN3A mAb or combination and then incubated with Vγ9Vδ2 T cells. Data are shown for E:T 30:1. The lysis is expressed as percentage of specific lysis. (D, E) Effect of anti-BTN3A mAb and ZOL on NK cells lysis compared to Vγ9Vδ2 T cells. Representative data from two independent experiments. Primary AML blasts lysis (n = 3) by (D) expanded Vγ9Vδ2 T cells from HV; (E) NK cells from HV isolated and treated O/N with IL2; DAUDI and K562 were used as positive controls respectively for Vγ9Vδ2 T cells and NK cells lysis. The lysis is expressed as percentage of specific lysis. Results were expressed as mean ± SEM and statistical significance was established using the non-parametric paired Wilcoxon U-test. *p < 0.05; **0.001 < p < 0.01; ***p < 0.001.
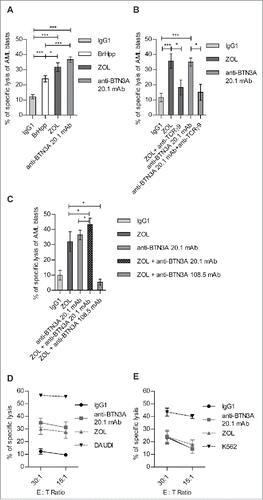
Figure 2. Triggering of BTN3A with 20.1 mAb enhances Vγ9Vδ2 T cells antitumor functions toward AML blasts. (A) Representative experiment showing trogocytosis of AML blasts from patient UPN01 by Vγ9Vδ2 T cells from HV (full gray histogram) and after 1 h in co-culture with IgG1 (dashed line) or anti BTN3A 20.1 mAb (full line). (B) Vγ9Vδ2 T cells trogocytosis (PKH67 MFI ± SEM) of AML blasts (n = 10). Cumulative data at 0 and 60 min from three different experiments are provided. (C) CD107a expression, TNFα and IFNγ production by Vγ9Vδ2 T cells from HV after a 4-h co-culture with AML blasts (n = 6).
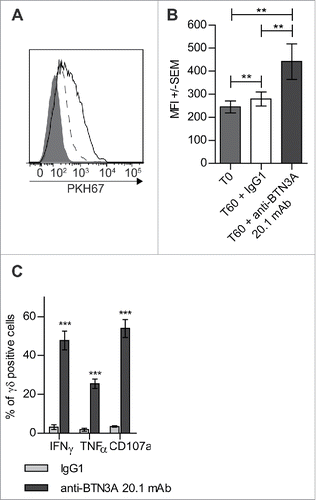
Figure 3. The 20.1 mAb triggers BTN3A on the surface of AML blasts. Primary AML blasts, target (T) lysis (n = 2) by Effector (E) Vγ9Vδ2 T cells from HV (n = 2) with IgG1 (full black line) or (i) after T pre-incubation with anti-BTN3A 20.1 mAb (anti-BTN3A + T) (full gray line) or (ii) after simultaneous incubation of E, T and anti-BTN3A 20.1 mAb (anti-BTN3A + E + T) (dashed gray line), or (iii) after E pre-incubation with anti-BTN3A 20.1 mAb (anti-BTN3A + E) (dashed black line). T or E cells were preincubated for 90 min with anti-BTN3A 20.1 mAb (10 μg/mL), and then extensively washed before E + T co-culture. Representative data from two independent experiments are depicted. Data are shown for E:T 1:1, 10:1, 30:1. The lysis is expressed as a mean percentage of specific lysis ± SEM and compared to DAUDI lysis.
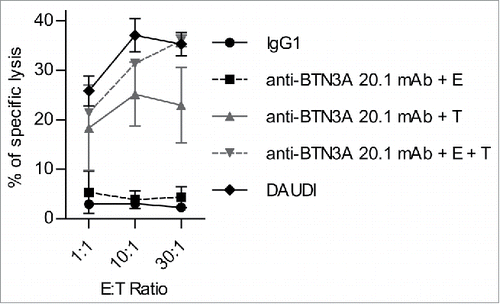
Figure 4. BTN3A molecules expression on primary AML blasts. (A) BTN3A molecules surface expression on primary AML blasts (n = 25). The global BTN3A surface expression was assessed by flow cytometry analysis. The results are expressed as Median Fluorescence Intensity ratio (rMFI) compared to control isotype. (B, C) BTN3A isoform expression on primary AML blasts. (B) qRT PCR analysis of total RNA isolated from primary AML blasts (n = 24). Data were normalized using GAPDH as endogenous control (ΔCt = Cttarget gene – CtGAPDH). Statistical significance was established using the non-parametric paired Wilcoxon U-test. ***p < 0.001. (C) Western-blot analysis of total protein extracts from primary AML blasts (n = 3). BTN3A knock-down HEK293FT (sh#284; clone#30) transiently transfected with BTN3A1m, BTN3A2m, BTN3A3m are used as size controls. Extracts were loaded in 10% SDS PAGE gel and membranes were hybridized with anti-BTN3A 20.1 mAb and anti-Grb2 as a loading control.
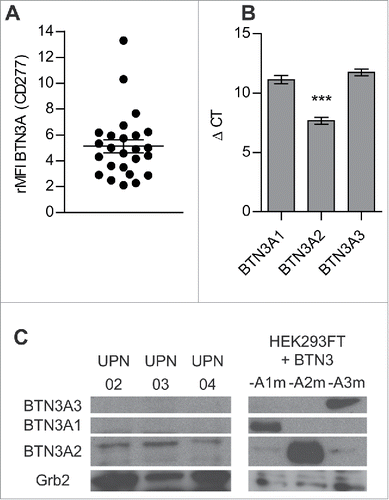
Figure 5. The sensitization of AML blasts by N-BP depends on monocytic component of blasts. (A, B) The percentage of monocytic component among AML blasts (SSCHigh CD45High) (n = 20) was assessed by flow cytometry. Representative dot plot of: (A) monocyticLow AML (UPN02) and (B) monocyticHigh AML (UPN10). (C) Linear regression of Vγ9Vδ2 T cells lysis of N-BP-treated AML blasts with respect to monocytic component (n = 20). The correlation was established using the non-parametric Spearman correlation coefficient (rs). ***p < 0.001. (D) Lysis of N-BP-treated blasts with low monocytic component (monocyticLow AML, % of SSCHigh CD45High<10) (n = 10) compared with the lysis of N-BP-treated blasts with high monocytic component (monocyticHigh AML, % of SSCHigh CD45High≥10) (n = 10) by Vγ9Vδ2 T cells. Results were expressed as mean ± SEM. Statistical significance was established using the Mann–Whitney test. 0.001 < **p < 0.01.
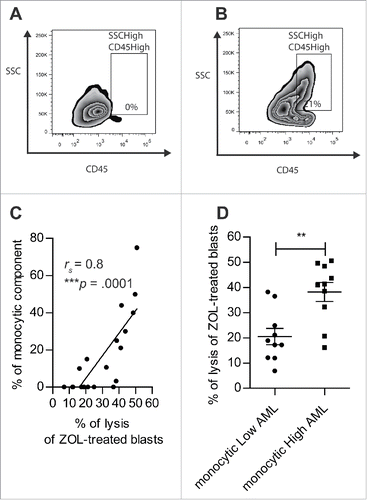
Figure 6. Anti-BTN3A 20.1 mAb can overcome N-BP poor sensitization of monocyticLow AML. (A) Specific lysis of monocyticLow AML blasts (n = 10) and (B) monocyticHigh AML blasts (n = 10) by Vγ9Vδ2 T cells from HV after treatment with IgG1, ZOL (O/N, 45 μM), or anti-BTN3A 20.1 mAb. Data are shown for E:T 30:1. Results were expressed as mean ± SEM and statistical significance was established using the non-parametric paired Wilcoxon U-test. *p < 0.05; 0.001 <**p < 0.01; ***p < 0.001.
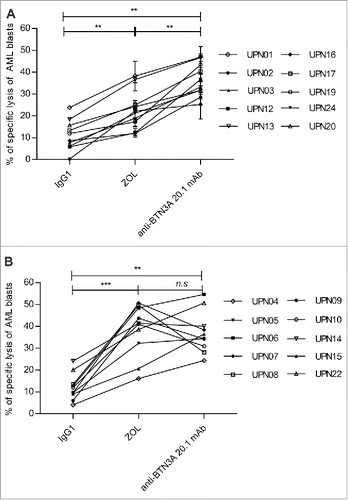
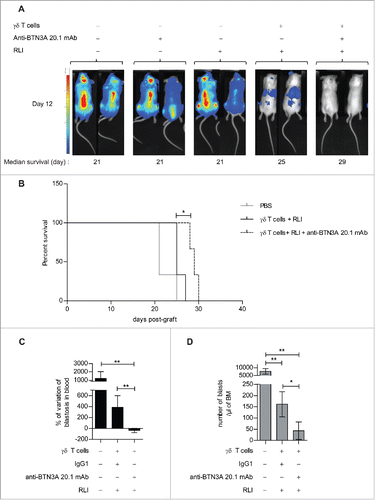
Figure 7. Evaluation of anti-BTN3A agonist mAb anti-leukemic activity in vivo in AML xenograft models. (A) (B) NSG mice (n = 15) were inoculated with luciferase-transfected U937 cells on Day 0. They were intravenously injected with PBS, anti-BTN3A mAb alone, RLI alone or 30 × 106 Vγ9Vδ2 T cells-plus-RLI or 30 × 106 Vγ9Vδ2 T cells-plus-RLI and anti-BTN3A 20.1 mAb. (A) Representative and comparative bioluminescent imaging of five groups of mice on Day 12 post-graft. Day 12 corresponds to the last bioluminescent measurement before first mice deceased. Medians of survival of each group of mice are notified below. (B) Median survival time was 21 d for the PBS control group (n = 3), 25 d for the group treated with Vγ9Vδ2 T cells and RLI (n = 3) and 29 d for the group treated with Vγ9Vδ2 T cells, RLI and anti-BTN3A 20.1 mAb (n = 3). Median survival times were compared using Log-Rank (Mantel-Cox) Test. *p < 0.05. (C, D) NSG mice (three groups of n = 6) were intravenously injected with primary AML blasts. Since the Week 8 post-graft, they were treated twice a week during two weeks with injections of 15 × 106 Vγ9Vδ2 T cells-plus RLI and either anti-BTN3A mAb or IgG1. Control mice were injected with PBS. Mice were sacrificed at the Week 11 post-graft. (C) Percentage of variation of the absolute number of blasts in blood quantified by Flow Cytometry before and 4 d after the end of the treatment. (D) Absolute number of blastic cells quantified by Flow Cytometry in harvested BM. Representative and cumulative data from two independent experiments. Mann–Whitney test was used to compare differences between treatments. *p < 0.05; 0.001 < **p < 0.01; ***p < 0.001.