Figures & data
Figure 1. Design and generation of DC targeting vaccine against HPV associated tumor. (A) Schematic diagram of the DC targeting tumor vaccine design and assembly. The αDEC205-SpyTag was prepared previously, and the HPV16 E743-62 polypeptide was fused to the C-terminus of SpyCatcherΔN. Once mixed, they conjugated to form an intact vaccine. (B) Vaccine production and purification. Purified αDEC205-SpyTag was mixed with SpyCatcherΔN-E7 at a molar ratio of 1:1.5 at 4°C for 2 h. The assembled αDEC205-Sc-E7 adduct was purified by Protein A chromatography. The purified adduct was analyzed by SDS-PAGE.
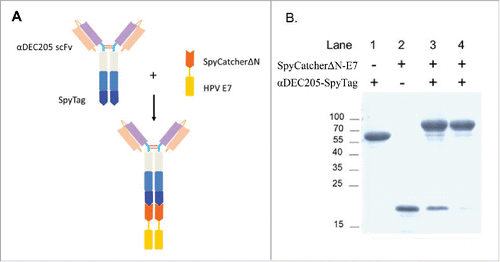
Figure 2. DC targeting vaccine efficiently targets DCs in vivo. C57BL/6 mice were injected with 200 pmol αDEC205-Sc-E7 protein, along with 30 μg CpG1826 and 30 μg Poly I:C as an adjuvant. Twenty hours later, cells were isolated from DLNs and stained. (A) Anti-MHC II and anti-CD11c antibodies were used to gate the DC populations. (B) and (C) Vaccine uptaken by DCs was measured by intracellular staining of the hIgG portion of the vaccine. Representative FACS plots (B) and the statistical analysis of the isotype-subtracted geometric MFI are shown (C). Data represent two independent experiments.
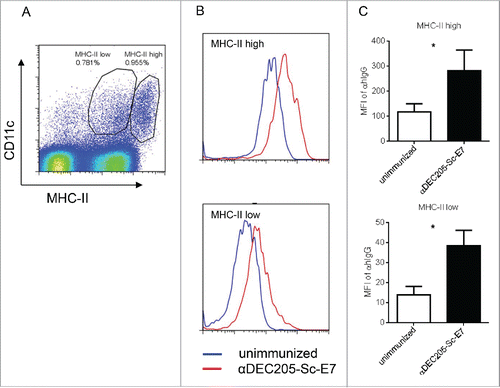
Figure 3. DC targeting tumor vaccine generates increased cytotoxic T cell response. C57BL/6 mice were subcutaneously immunized with 120 pmol αDEC205-Sc-E7 or Sc-E7, with 30 μg CpG1826 and 30 μg Poly I:C as adjuvant. (A) Seven days later, cells were isolated from the DLNs and restimulated with HPV16 E749-57 peptide (5 μg/mL) for 6 h in the presence of brefeldin A. (B) The frequency of IFNγ+ cells among CD8+ T cells was analyzed. Mean ± SD (n = 5). **p < 0.01. (C) and (D) Fourteen days later, 5 × 105 TC-1 cells were injected subcutaneously, then tumor volumes were monitored. The growth curve is shown in panel C and the survival curve is shown in panel D. (n = 5–6). **p < 0.01, ***p < 0.001.
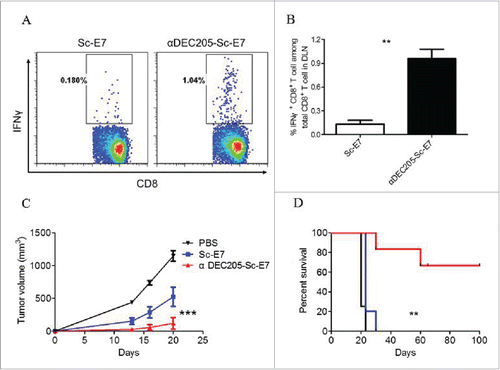
Figure 4. DC targeting tumor vaccine can efficiently inhibit tumor growth. (A) and (B) C57BL/6 mice were inoculated with 5 × 104 TC-1 cells subcutaneously at right flank. Nine and fourteen days later, mice were injected with 120 pmol Sc-E7 or αDEC205-Sc-E7, respectively. CpG & Poly I:C were used as adjuvant as before. The tumor size was monitored. The growth curve is shown in panel A and the survival curve is shown in panel B. (n = 5–6). **p < 0.01, ***p < 0.001.
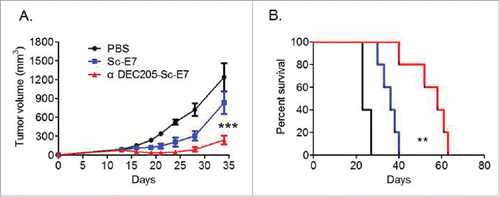
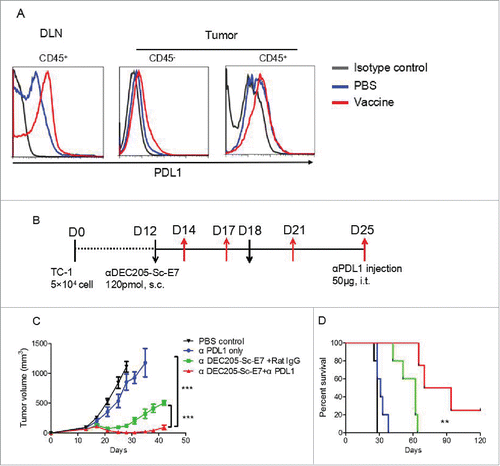
Figure 5. Vaccination increases PD-L1 and anti-PD-L1 blockade can synergize with DC targeting tumor vaccine to control tumor growth. C57BL/6 mice were inoculated with 5 × 104 TC-1 cells subcutaneously at right flank. (A) Nine days later, mice were immunized with αDEC205-Sc-E7 protein, together with 30 μg CpG and 30 μg Poly I:C as adjuvant. Three days later, PD-L1 expression on cells from DLNs and tumors (A) was analyzed. (B) For combinational therapy, 12 and 18 d after tumor inoculation, mice were injected with 120 pmol αDEC205-Sc-E7. Meanwhile, anti-PD-L1 was administered intratumorally on days 14, 18, 21 and 25; Rat-IgG was used as an isotype control. The tumor size was monitored. (C) and (D) The growth curve is shown in panel C and the survival curve is shown in panel D. (n = 5–6). **p < 0.01, ***p < 0.001.