Figures & data
Figure 1. Abundance of tumor infiltrating lymphocytes in early stage MSS colorectal cancers track with relapse-free survival. (A) CD8+ cells were evaluated in two cohorts of patients (Series 1- RMH), one that had tumor relapse (n = 10) that other without (n = 10). The area of positive CD8+ IHC signal was found to the significantly higher in relapse-free patients (two-way t-test). (B) CD45RO+ cells IHC showed no significant difference between the two cohorts. (C) Data from (A) were used to calculate the statistical median which was used to partition with an area above 9.5% and those 9.5% and below. Relapse-free patients were found to have significantly higher CD8+ cells that tracked with relapse-free survival (Log-rank, Mantel–Cox test). (D) Data from (B) were used to calculate the median percentage of CD45RO+ cells whereby tumors with a median above 25.3% and those below were indistinguishable in terms of outcome. (E) CD8+ cells were evaluated in two cohorts of patients from a separate hospital (Series 2- SJOG), one that had tumor relapse (n = 11) that other without (n = 9). The area of positive CD8+ IHC signal was found to the significantly higher in relapse-free patients (two-way t-test, n = 10). (F) Data from (E) were used to calculate the statistical median which was used to partition with an area above 27.5% and those below whereby the relapse-free patients were found to have significantly higher CD8+ cells tracked with relapse-free survival (Log-rank, Mantel–Cox test). (G) Data from Series 1 and 2 (Panels A and E) were pooled and the median recalculated as above and whereby tumors with a median above 12.5% and those below found to have significantly higher CD8+ cells tracked with relapse-free survival (Log-rank, Mantel–Cox test). (H) When the percentage of CD8+ plus CD45RO+ cells were evaluated those tumors where high expression was calculated patients were found to be essentially relapse-free.
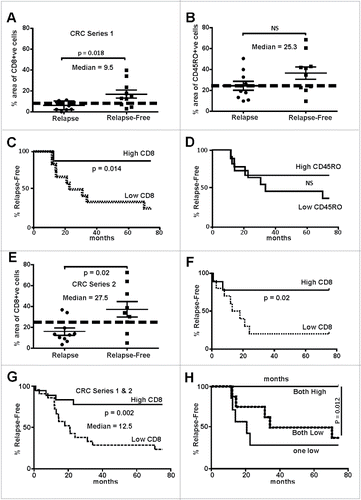
Figure 2. MYB and GRP78 expression is generally higher in colorectal tumors than in normal adjacent mucosa. (A) RNAseq was used to determine significantly higher GRP78 and MYB expression in CRC (T) compared the matched normal mucosa (N). (Two-way, t-test, n = 14 plus 2 addition CRC samples). (B) IHC for MYB and GRP78 antigens in the same region of an early stage MSS CRC.
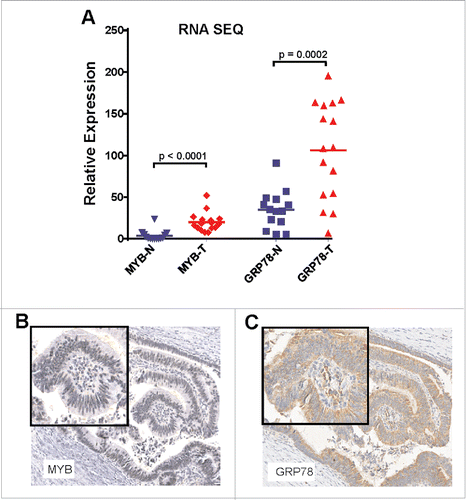
Figure 3. MYB and GRP78 expression tracks with CRC patient outcome. (A, B) IHC Histoscore was used to determine high and low MYB expression in relapse (n = 21) and relapse-free (n = 19) MSS CRC showing that high MYB tracks with poor outcome as well as tumor relapse (C, D) IHC Histoscore was used to determine high and low GRP78 expression in relapse and relapse-free CRC showing that high GRP78 tracks with poor outcome as well as relapse-free survival.
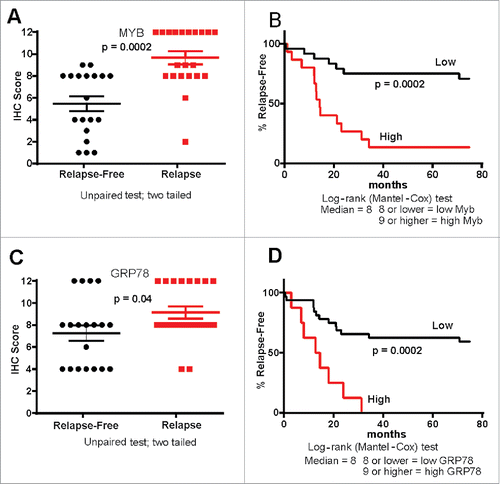
Figure 4. GRP78 expression is higher in CRC where CD8+ TILs cells are absent. (A) Two consecutive series of IHC representative of a relapse-free and (B) a relapse patient CRC are shown. β-catenin demarks tumor epithelium as well as invading tumor cells (open arrow) along with low GRP78 which is associated with greater CD8+ TILs. Black arrows identify CD8+ TILs.
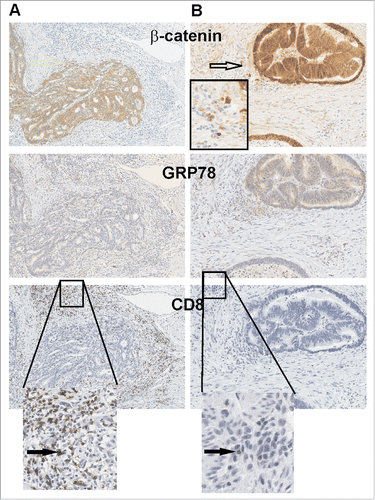
Figure 5. MYB overexpression increases GRP78. (A) Green fluorescent protein (Gfp) gene reporter expression in mouse CT26 CRC cells transduced with a MYB-expressing retrovirus is shown. (B) Induction of endogenous Grp78 mRNA expression in MYB-GFP-CT26 cells compared to parental cells. (C) Tumor formation kinetics (volume) in parental verses MYB-GFP-CT26 cells was determined. (D) IHC for GRP78 in parental verses MYB-GFP-CT26 including secondary antibody control (2nd Ab). (E) Percentage area of tumor expressing GR78 quantified by MetamorphR analyses is shown.
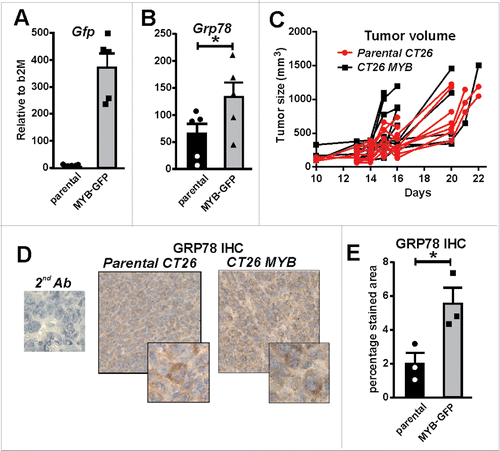
Figure 6. Increased MYB is associated with suppressed immune activation. (A) CD8+ TILs were isolated from tumors generated by parental and MYB-GFP-CT26 cells and assessed for activation marker CD44. (B) Tumor draining lymph nodes were isolated on the basis of CD4+ or CD8+ expression and then evaluated for T-cell activation markers CD62L and CD44. Significantly reduced activation status was observed in mice bearing MYB-GFP-CT26 tumors; (*p < 0.05, t-test). (C) Tumor growth was indistinguishable when CT26 cells with either MYB-GFP or GFP were compared following doses of isotype control antibodies (200 mg IP) at day 5, 10 and 15 post tumor cell inoculation (arrows). (D) When mice bearing CT26 MYB-GFP cells were treated with anti-PD-1 antibodies (200 mg IP) at day 5, 10 and 15 (arrows) 3/6 mice escaped growth inhibition compared to 0/6 mice in the CT26-GFP control group.
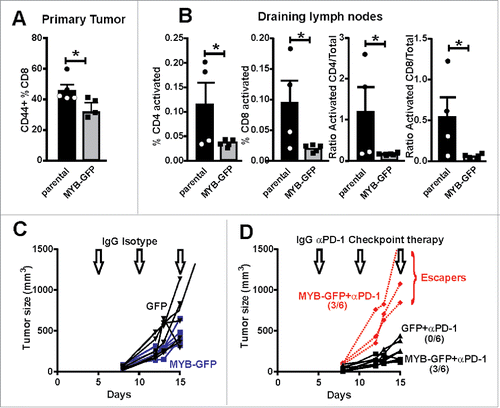
Table 1. Patient and tumor characteristics.
