Figures & data
Figure 1. PD1 blockade prolonged survival in an orthotopic PDA murine model and its effect was dependent upon innate and adaptive components of the immune response. (A) Flow cytometric analysis of KPC cells growing in culture demonstrating endogenous expression of PD-L1. (B) Kaplan–Meier curve showing survival benefit of single-dose administration of α-PD1 (n = 12) compared to IgG isotype (n = 11) or PBS vehicle control (n = 9). (C) Flow cytometric analysis of PBMC demonstrating >80% depletion of each immune cell subset 24 h after antibody administration. (D) Mice received IgG isotype control (n = 9) or α-PD1 (n = 12) alone or in combination with individual depletion antibodies: α-Gr1 (n = 10), α-NK (n = 7), α-CD4+ (n = 8) or α-CD8+ (n = 12). Depletion antibodies were continuously administered every 3 d to prevent immune cell repopulation. Results are expressed as percentage of change in bioluminescence signal intensity by measuring luciferase activity using IVIS at day 0 versus day 15. Change in bioluminescent signals were compared to α-PD1 and statistical significance calculated using non-parametric Mann–Whitney test. Each symbol represents an individual mouse. Plots are showing the combined results of at least two independent experiments.**p < 0.01, ***p < 0.001.
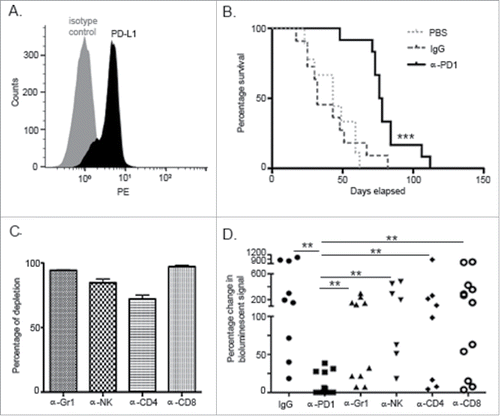
Figure 2. Treatment with α-PD1 induced short but not long-term transferrable protective immunity. Kaplan–Meier curve showing survival benefits of adoptively transferring total splenocytes obtained from tumor-bearing donor mice at (A) day 3, (B) 7, or (C) 28 after single-dose treatment with α-PD1 (day 3: n = 9; 7: n = 8, 28: n = 8) or IgG isotype control (day 3: n = 8; 7: n = 7, 28: n = 6) into cyclophosphamide pre-conditioned tumor-bearing recipient mice. (D) Mice received α-PD1 (n = 8) alone or in combination with depletion antibodies: α-Gr1+α-NK (n = 7) or α-CD4+α-CD8+ (n = 8). Statistical significance was calculated using non-parametric Mann–Whitney test. Results are expressed as percentage of change in bioluminescence signal intensity by measuring luciferase activity using IVIS at day 0 versus day 15. Each symbol represents an individual mouse. Plots are showing the combined results of at least two independent experiments. N.S. = not significant, *p < 0.05.
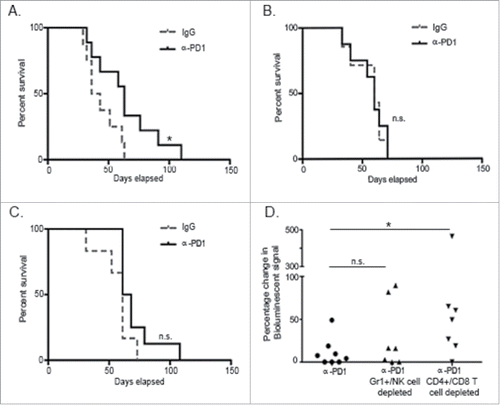
Figure 3. α-PD1 exhibited prolonged binding to CD11+Gr1+ cells and NK cells present in the spleen and tumor. Single-cell suspensions obtained from both spleens and tumors of mice after 3 or 7 d treatment with IgG isotype control (day 3: n = 4, 7: n = 5) or α-PD1 (day 3: n = 4, 7: n = 4) were subjected to flow cytometric analysis. Percentage of antibody that remained bound to (A) CD11b+Gr1+ cells or (B) NK cells. (C) Total percentage of CD11b+Gr1+ cells were estimated in the live CD45+ gated population and (D) of NK cells in the live CD45+CD3− gated population. (E) Baseline endogenous expression of PD1 receptor in CD11b+Gr1+ cells and NK cells at day 0 of treatment. Each symbol represents and individual mouse. Results from two independent experiments were combined. *p < 0.05, ***p < 0.001 by 2-tailed unpaired test.
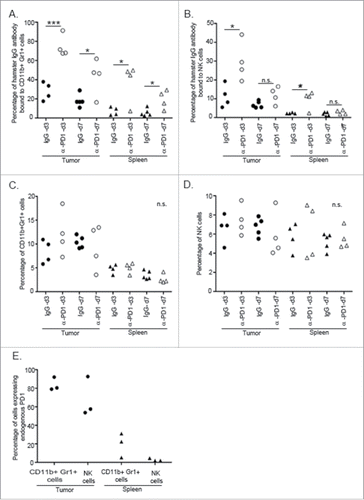
Figure 4. Adoptive transfer of purified Gr1+ cells and/or NK cells failed to control tumor progression in untreated recipient mice. Kaplan–Meier curve showing no survival benefit of adoptively transferred NK cells and/or Gr1+ cells isolated from total splenocytes at day 3 after treatment of tumor-bearing donor mice with α-PD1 (NK: n = 6; Gr1+: 5; NK+Gr1+: n = 7) versus IgG isotype control (NK: n = 4; Gr1+: 5; NK+Gr1+: n = 6). Cells were transferred into untreated tumor-bearing recipient mice pre-conditioned with cyclophosphamide. Experiments were repeated at least four times and the results combined.
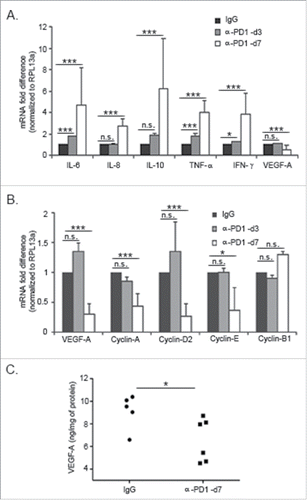
Figure 5. Inhibition of PD1 modulated the expression of pleiotropic cytokines and cell cycle-related genes in the TME. Single-cell suspensions were obtained from tumors harvested at day 3 or 7 after treatment with IgG isotype control or α-PD1. RT-qPCR showing changes in (A) in cytokine expression in the CD45+ tumor-infiltrating cells or (B) in VEGF-A and G1/S cyclins in the CD45− tumor cell enriched fraction. Results were normalized to RPL13a housekeeping gene and expressed as a fold difference relative to the IgG isotype control expression levels. Each bar represents the combined result of three independent experiments, containing pooled tumor samples from 2–3 mice in each experiment. Prior to normalizing the data, results were analyzed using the comparative ΔΔCT method and significance determined by using ANOVA followed with Bonferroni correction. (C) VEGF-A levels were determined by ELISA assay using fresh tumor tissue lysates obtained from mice treated with α-PD1 (n = 6) or IgG isotype control (n = 5) for 7 d. Each symbol represents an individual mouse. Comparison of VEGF-A protein levels between α-PD1 versus IgG isotype control treated mice was calculated using non-parametric Mann–Whitney test. N.S. = not significant. *p < 0.05, ***p < 0.001.