Figures & data
Figure 1. CXCL16 expression on DCs, B cells and macrophages following iNKT cell stimulation with α-GalCer. Spleen and liver cells were harvested 0–72 h after mice were treated intraperitoneally with the glycolipid α-GalCer (4 μg). CXCL16 expression was analyzed on different immune cells by flow cytometry. (A) Representative histograms show CXCL16 expression on liver DCs (CD11c+ MHCII+), B cells (CD19+ B220+) and macrophages (CD11b+ F4/80+) under basal conditions and 18 h after α-GalCer treatment. (B) Aggregate data show CXCL16 expression on liver and spleen cell populations under basal conditions and 18 h after α-GalCer treatment (n = 3–8 per group). (C) Time course of CXCL16 expression on splenic DCs from wild-type and Jα18−/− mice following glycolipid stimulation. (n = 4 per time point). *p < 0.05 compared to baseline. †p < 0.05 compared to wild-type.
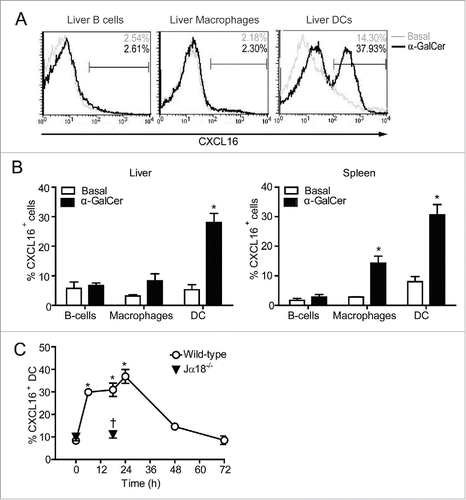
Figure 2. In vitro iNKT cell activation in the presence and absence of CXCL16. Liver mononuclear cells were cultured for 2 h in wells coated with 0, 1 or 5 μg/mL anti-CD3, with or without 100 ng/mL of recombinant CXCL16. iNKT cells (CD1d tetramer+ TCRβ+) were stained to examine intracellular (A) IFNγ and (B) IL-4 production, and cell surface expression of (C) CD40L and (D) CD69 by flow cytometry (n = 3 per group). Sorted human iNKT cells (5 × 104 TCRβ+Vα24Jα18+) were cultured in wells coated with 0, 1 or 5 μg/mL anti-CD3, with or without 100 ng/mL of recombinant CXCL16. After 24 h supernatants were harvested to measure (E) IFNγ and (F) IL-4 production (n = 5 healthy donors). *p < 0.05 compared with anti-CD3 alone.
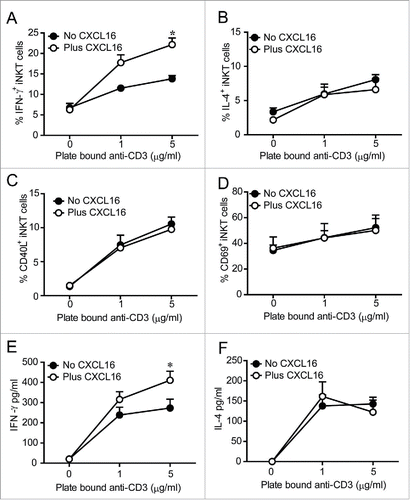
Figure 3. In vitro cytokine responses of iNKT cells stimulated with glycolipid-loaded CXCL16hi or CXCL16neg DCs. CD11c+ DCs were enriched from the spleen by magnetic sorting and loaded overnight with α-GalCer (200 ng/mL). DCs were sorted into CXCL16hi and CXCL16neg subsets and incubated with sorted iNKT cells (CD1d-tetramer+ TCRβ+) for 24 h, at a ratio of 1:2, DCs to iNKT cells. Cytokine levels in culture supernatants were examined using a cytokine array (n = 5–6 per group). *p < 0.05 compared with CXCL16hi DCs.
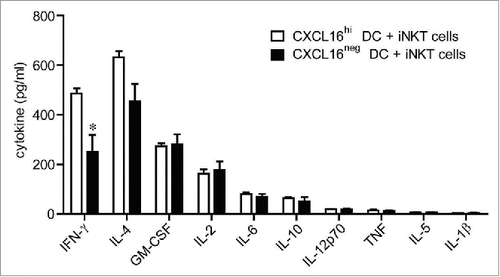
Figure 4. In vivo cytokine responses following adoptive transfer of α-GalCer-loaded CXCL16hi or CXCL16neg DCs. CD11c+ DCs were enriched from splenocytes by magnetic sorting and loaded overnight with α-GalCer (200 ng/mL). DCs were sorted into CXCL16hi and CXCL16neg subsets and injected i.v. into wild-type or Jα18−/− mice. Unloaded DCs and Jα18−/− mice were used as controls. Serum cytokine levels were analyzed via a multiplex cytokine array. (A) IFNγ, IL-4, and IL-12p-70 levels were measured at 2, 6 and 18 h after DC transfer (n = 5–10 per group). *p < 0.05 compared with 2 h time point, †p < 0.05 compared to CXCL16hi DC. (B) Representative plots showing expression of CD86, CD80, CD40, I-A, and CD1d on CXCL16hi DCs and CXCL16neg DCs following isolation and after overnight culture. Receptor expression is also shown for the CD86hi gated population. (C) Serum levels of IFNγ and IL-4 were measured by ELISA 18 h after adoptive transfer of α-GalCer-loaded CD86hi CXCL16hi or CD86hi CXCL16neg DCs. (n = 4 per group). *p < 0.05 compared with CXCL16hi DC. (D) Serum IFNγ, IL-4, and IL-12p70 levels were measured by ELISA 18 h after adoptive transfer of splenic CXCL16+/+ or CXCL16−/− DCs (n = 6 per group). *p < 0.05 compared to CXCL16+/+ DCs.
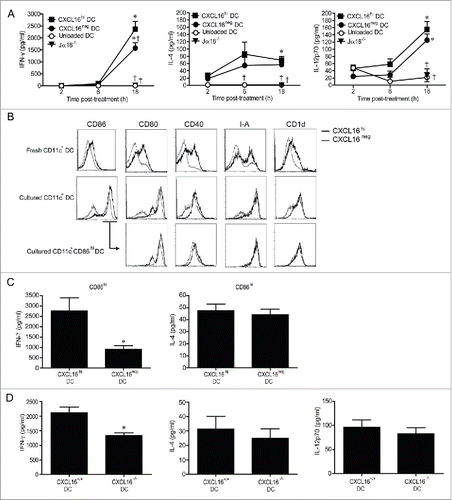
Figure 5. In vivo profiling of intracellular IFNγ and T-bet expression in iNKT cells following adoptive transfer of α-GalCer-loaded CXCL16+/+ or CXCL16−/− DCs. Mice were stimulated by adoptive transfer of α-GalCer-loaded CXCL16+/+ or CXCL16−/− BMDCs. The number of (A) total iNKT cells, (B) IFNγ+ iNKT cells and (C) T-bet+ iNKT cells was examined by flow cytometry 2 h or 72 h later (n = 3–6 per group). *p < 0.05 compared to unloaded DCs, †p < 0.05 compared to CXCL16+/+ DCs.
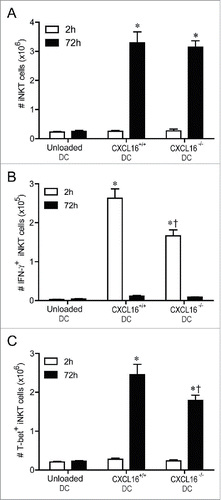
Figure 6. Expression and role of CXCR6 in NK cell transactivation. (A) Surface expression of CXCR6 was examined on NK cells (NK1.1+ TCRβ−) and iNKT cells (CD1d-tetramer+ TCRβ+) isolated from spleen and liver using a chimeric CXCL16-Fc construct (n = 4 per group). (B and C) Expanded iNKT cells (CD1d tetramer+ TCRβ+) from wild-type donor mice were adoptively transferred (i.v. 1 × 107) into recipient Jα18−/− or Jα18−/− CXCR6−/− mice. Twenty-four hours later, control and iNKT cell-reconstituted mice were stimulated with α-GalCer-loaded CXCL16hi BMDCs or unloaded control DCs (i.v. 2 × 105). (B) Serum cytokine levels and (C) NK cell intracellular IFNγ staining were examined 18 h following stimulation (n = 3 per group). *p < 0.05 compared to no DC stimulation.
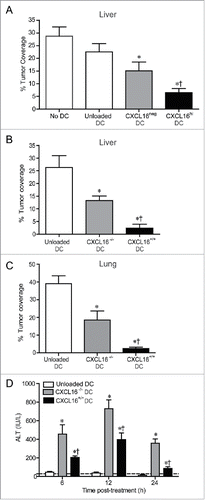
Figure 7. Control of metastatic B16 melanoma lesions in the liver and lung via transfer of glycolipid-loaded DCs. (A) To induce liver metastasis, wild-type mice were inoculated in the spleen with 2.5 × 105 B16 melanoma cells. Five days later, mice were injected i.v. with 2 × 105 α-GalCer-loaded CXCL16hi or CXCL16neg BMDCs, or unloaded control BMDCs generated from wild-type mice (n = 14–20 per group). *p < 0.05 compared to unloaded DCs, †p < 0.05 compared to CXCL16neg DCs. (B) In independent experiments, 2 × 105 α-GalCer-loaded BMDCs generated from wild-type or CXCL16−/− mice were delivered 5 d after B16 inoculation (n = 9–10 per group). (C) To induce lung metastasis, wild-type mice were inoculated i.v. with 2.5 × 105 B16 melanoma cells. Three days later, mice were injected i.v. with 2 × 105 α-GalCer-loaded BMDCs generated from wild-type or CXCL16−/− mice (n = 4–6 per group). Liver and lung metastasis were examined 14 d after tumor cell inoculation using image analysis software to calculate tumor coverage. (D) Serum ALT levels were measured in tumor-bearing mice following administration of wild-type or CXCL16−/− DCs (n = 4–6 per group). *p < 0.05 compared to unloaded DCs, †p < 0.05 compared to CXCL16−/− DCs.