Figures & data
Figure 1. Myeloid PTEN-deficiency increases tumor burden and mortality. (A) timeline of AOM/DSS treatment to induce colitis-associated colon cancer (CAC), (B) Representative H&E-stained sections of swiss rolls (small and large intestines) of PTENfl/fl and PTENfl/fl LysM cre mice and quantification of tumor number and area, n = 7–11, *p <0.05. (C) Weight change during CAC development of one representative experiment n = 6–8, (D) representative pictures of unflushed colons and quantification of colon length (n = 5–12). (E) Kaplan–Meier estimator of survival during CAC development (n = 30–46), *p < 0.05.
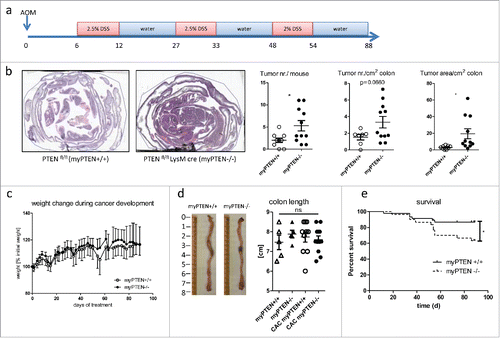
Figure 2. Myeloid PTEN-deficiency increases tumor growth of B16-F10 melanoma. (A) Representative bioluminescence images of myPTEN+/+ and myPTEN−/− mice on day 2, day 6 and day10 after s.c. B16-F10-Ova-Luc injection, (B) Quantification of tumor growth by bioluminescence imaging of luciferase activity, n = 10–14. (C) Weight change of B16-injected mice, values are expressed as percent initial weight before tumor inoculation, n = 4 – 7.
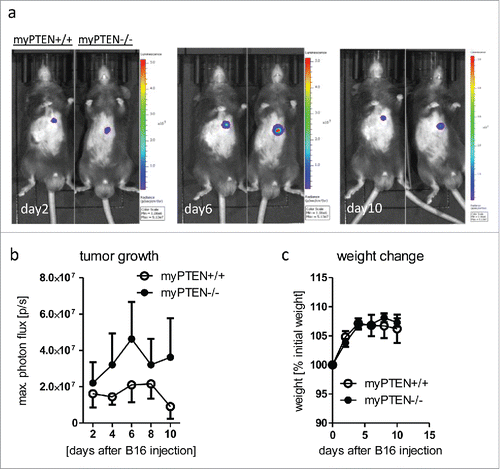
Figure 3. Myeloid PTEN-deficiency increases CD8a+DCs in the spleen. (A) Representative dot plots for identification of CD8α+DCs (gated on live, CD45+ CD3− cells). (B) Quantification of myeloid subsets (CD11c+CD11b+; CD11c+CD11b− and CD11c+CD8a+ cells in spleen-SPL, colon lamina propria-CLP and colon epithelium-CEP), values are expressed as % of live cells, n = 4–6, *p < 0.05, **p < 0.01. (C) Representative density plot for identification of CD11c+ MHCII+PDL-1+ and PDL-2+ cells, respectively (gated on live, CD45+CD11c+CD11b−). (D) Quantification of myeloid MHCII+ PDL1+ and PDL2+ cells (gated on live, CD45+) in the spleen, values are expressed as % of live cells, n = 4–6, *p < 0.05, **p < 0.01. (E) Splenic CD11c+ MHCII+APCs co-express CD8α and PDL-1 or PDL-2, respectively, (F) Myeloid subsets and immune checkpoint inhibitors in naive healthy mice, gating as in (A) or (C), respectively, values are expressed as % of live cells, n = 4–5, *p < 0.05.
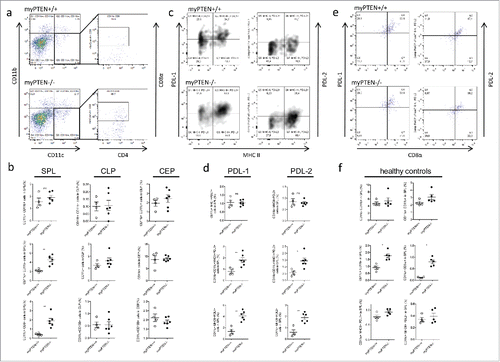
Figure 4. Transcriptome analysis of enriched splenic CD11c+ cells. (A) Heatmap of differentially expressed genes considering a p value of <0.05 as significantly changed. (B) Selection of most significantly changed Kegg pathways containing differentially expressed genes in splenic CD11c+ cells from myPTEN−/− and myPTEN+/+ mice; list is sorted according to p value. (C) Fold changes of transcription factors involved in DC development and function. (D) Fold changes of selected genes know to be differentially regulated between myPTEN−/− and myPTEN+/+ in CD11b+ and CD11c+ cells.
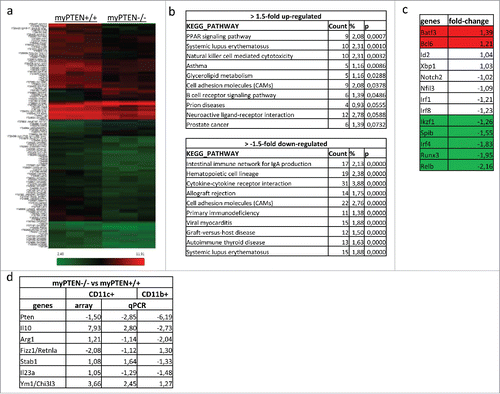
Figure 5. Myeloid PTEN deficiency induces hypo-responsiveness in T-cells. (A) Quantification of flow cytometry analysis of (live, CD45+ CD3+) CD4+ T-helper cells and CD8+ CTLs in spleen (SPL), colon lamina propria (CLP) and colon epithelium (CEP) of CAC mice, values are expressed as % of live cells, n = 4–6, *p < 0.05; (B) quantification as in (A) of healthy animals, values are expressed as % of live cells, n = 4–5; (C) proliferation of total splenocytes of myPTEN+/+ and myPTEN−/− mice after ex vivo culture for 3 d in the presence of activating anti-CD3ϵ measured by the incorporation of radioactive 3H-thymidine, n = 8, *p < 0.05; (D) Proliferation of CFSE-labeled OT-I T-cells 3 d after i.v. transfer into myPTEN+/+ and myPTEN−/− CAC mice. Mice were immunized with LPS/Ova one day after i.v. transfer, proliferation was assessed by CFSE dilution and is shown as % OT-I T-cells in SPL and MLN, % proliferating OT-I T-cells and MFI of CFSE, n = 2–5, values were measured in triplicates, **p < 0.01, ***p < 0.001; (E) representative CD3-IHC and (F) F4/80 staining of paraffin-embedded sections of colon swiss rolls from myPTEN+/+ and myPTEN−/− CAC mice and quantification of positive stained areas per tumor area, values are expressed as % of tumor area, n = 15–17, **p < 0.01, ***p < 0.001.
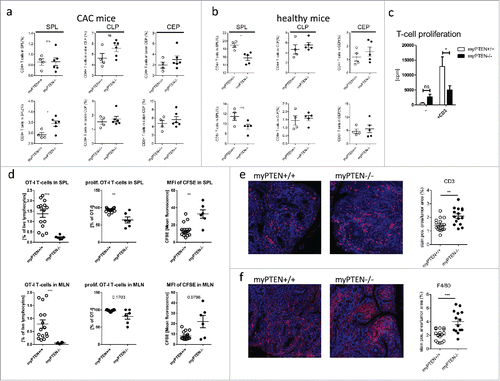
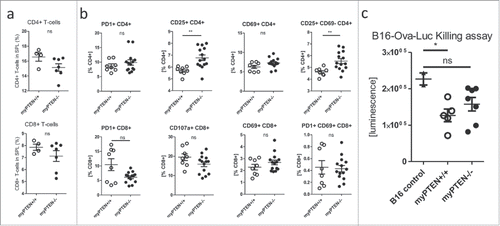
Figure 6. Myeloid PTEN deficiency induces regulatory T-cells. (A) Quantification of flow cytometry analysis of (live, CD45+ CD3+) CD4+ T-helper and CD8+ CTLs in the spleens of myPTEN+/+ and myPTEN−/− mice 10 d after s.c. B16 melanoma injection, values are expressed as % of live cells, n = 4–7; (B) Quantification of flow cytometry analysis of activation markers PD-1, CD25, CD69 and CD107a in CD4+ T-helper and CD8+ CTLs in the spleens of myPTEN+/+ and myPTEN−/− mice 10 d after s.c. B16 melanoma injection, values are expressed as % of CD4+ or CD8+, respectively, n = 8–14, *p < 0.05, **p < 0.01; (C) in vitro killing of B16-Ova-Luc cells was assayed by measurement of remaining luciferase activity specific for transduced melanoma cells after co-culture for 1 h with LPS/SIINFEKL-primed splenocytes from B16-injected myPTEN+/+ or myPTEN−/− mice as in (A) and (B), respectively, values represent arbitrary luminescence values; n = 2–7, *p < 0.05, **p < 0.01.