Figures & data
Figure 1. rED44Her2 and rED44Her2-FrC conjugate vaccines induce potent humoral immunity against rED44Her2 and native rHer2, and protect against challenge with the TUBO mammary carcinoma. (A) Schematic representation of the Her2 molecule (highlighting the ED44Her2 fragment) and the ED44Her2-FrC conjugate vaccine design. Diagram is representative of both the rat and human forms of Her2 and ED44Her2. (B) BALB/c wild type mice were primed and boosted 3 weeks later with rED44Her2, rED44Her2-FrC or the plant expressed control vaccine, all in alum, or the EC-TM DNA vaccine (n = 5 per group). Antibody specific for the rED44Her2 fragment was measured by ELISA. Serum was collected after priming, at week 3, and at week 5 which was 2 weeks after the boost. (C) Antibody able to bind native rHer2 expressed on the surface of TUBO cells was measured by flow cytometry at week 5 and quantified using internal standards. (D) Correlation between the levels of anti-rED44Her2 antibody, as measured by ELISA, and anti-rHer2 antibody, as measured by binding to native rHer2 at the cell surface. Data from the rED44Her2 vaccine at week 5. r = the Spearman's rank correlation coefficient. (E) 6 weeks after the booster injection (week 9) mice were challenged with the transplantable TUBO mammary carcinoma, and culled when tumors reached 15 mm diameter. In (B) and (C) each bar represents medians with individual mice displayed as dots. Mann–Whitney statistics are shown. Log-rank (Mantel–Cox) test results shown in (E). ns = p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001. For (B) and (D) data from two experiments has been combined. For (C) and (E) one representative experiment of three is shown.
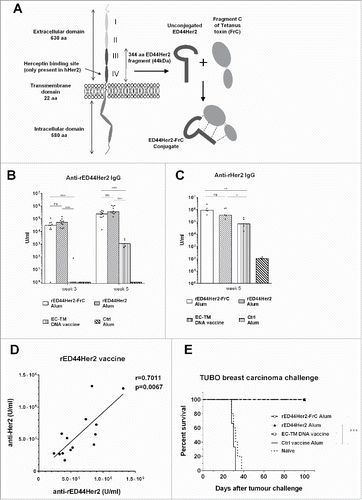
Figure 2. The rED44Her2-FrC conjugate vaccine affords superior protection against spontaneous mammary carcinoma in the BALB-neuT model, and induces high levels of anti-rED44Her2 antibody. (A) BALB-neuT mice aged 10 weeks were vaccinated with rED44Her2, rED44Her2-FrC or the irrelevant control protein vaccine, in alum, or the EC-TM DNA vaccine. They were then boosted every 3 weeks (see arrows on graph). Development of spontaneous tumors was observed and mice terminated when the sum total mean diameter of tumors exceeded 15 mm. (B) Serum samples from mice in (A) were tested for rED44Her2-specific antibody at the indicated time points by ELISA. In (A) log-rank (Mantel–Cox) test results are shown, and in (B) median values are plotted as bars, with Mann–Whitney statistics. Ns = p > 0.05, *p < 0.05, **p < 0.01, ****p < 0.0001. Data combined from two experiments.
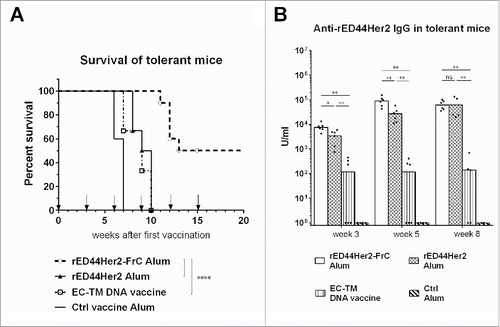
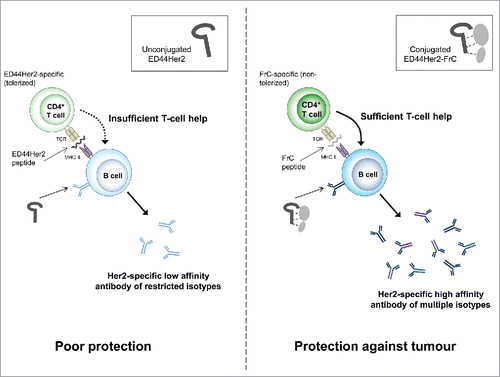
Figure 3. The rED44Her2-FrC conjugate vaccine induced potent CD4+ Th cell responses in BALB-neuT tolerant mice, which correlated with induction of a broad range of IgG antibody isotypes, and antibody of high affinity. (A) Tolerant BALB-neuT mice were vaccinated with either the rED44Her2-FrC conjugate vaccine, the rED44Her2 vaccine or a control vaccine (3–5 mice per group from three independent experiments). Spleens were taken at day 14 and the number of CD4+ Th cells secreting cytokines IL-2, IL-4 or IFNγ were measured by ELISpot after restimulation with rED44Her2 protein, FrC protein or an irrelevant control protein (Ovalbumin). The mean spot forming units (SFU) per 106 lymphocytes (with SEM) from each group of mice is plotted, with non-specific background values (restimulation with media alone) subtracted. The cut-off line represents 2 times responses from mice vaccinated with the control vaccine, or responses to the control protein, whichever was highest. Mann–Whitney statistics are shown, between rED44Her2 stimulated groups. Ns = p > 0.05. (B) Antibody isotypes (IgG1, IgG2a and IgG2b) were measured by ELISA in BALB-neuT tolerant mice at week 5, following both priming and boosting with the rED44Her2-FrC or rED44Her2 vaccines, in alum. (C) Antibody affinity was measured using a chaotropic ELISA. Affinity index indicates the concentration of chaotropic agent required to reduce antibody binding by 50%, with higher affinity antibody requiring a higher concentration to disrupt binding. Serum was taken at week 5, as in (B). In (B) and (C) medians are plotted with data pooled from three independent experiments with similar results. Mann–Whitney statistics are shown, with *p < 0.05, **p < 0.01.
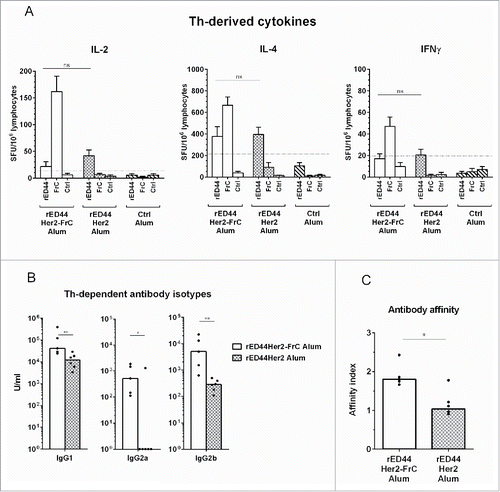
Figure 4. TLR4 agonist MPL cannot compensate for lack of T-cell help in terms of antibody affinity, IgG isotype induction or protection from spontaneous tumor development. BALB-neuT mice (n = 3–5 per group) were vaccinated with either rED44Her2 or rED44Her2-FrC, in alum or in combination with alum plus MPL. Serum was taken after priming, at week 3, or after boosting, at week 5. (A) Anti-rED44Her2 antibody levels measured by ELISA. (B) IgG antibody affinity from week 3 samples was measured by a chaotropic ELISA, and the affinity index calculated. (C) Levels of IgG isotypes were measured from serum samples taken at week 5. Medians are plotted, with Mann–Whitney statistics shown. Ns = p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001. (D) Survival of BALB-neuT mice from the spontaneous development of mammary tumors was followed after the above vaccination protocol. Mice were culled when total mean diameter of the tumors reached 15 mm. Log-rank (Mantel–Cox) statistics shown. Ns = p > 0.05, *p < 0.05. Due to the limited number of BALB-neuT mice available, results from two experiments showing the same trends have been combined in (A), (B), (C) and (D).
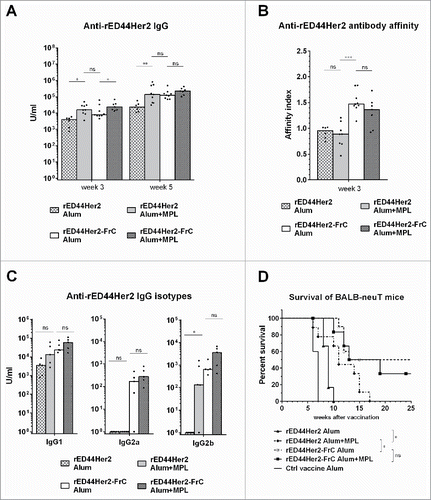
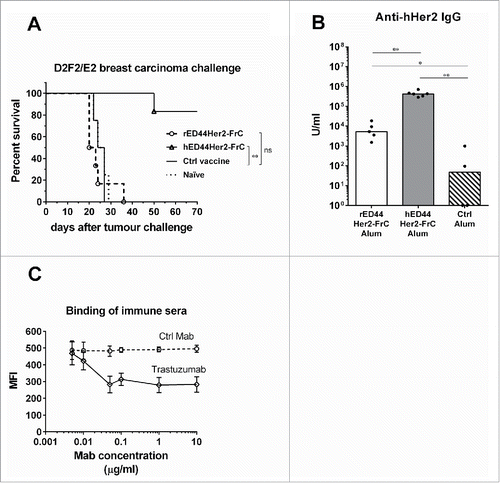
Figure 5. Evaluation of the rat and human ED44Her2-FrC conjugate vaccines in the D2F2/E2 model which expresses hHer2. (A) Wild type BALB/c mice (n = 4–6 per group) were vaccinated twice with the vaccines indicated (at week 0 and week 3) and at week 8 were challenged with 5 × 105 D2F2/E2 mammary carcinoma cells. Survival was followed from the day of tumor challenge, with mice culled when total tumor diameter reached 15 mm. Log-rank (Mantel–Cox) statistics are shown, ns = p > 0.05, **p < 0.01. (B) Levels of anti-hHer2 antibody were quantified by analysis of binding to native hHer2 expressed on the surface of D2F2/E2 cells by FACS. Quantification was relative to internal standards. Bars are median values, with Mann–Whitney statistics shown, *p < 0.05, **p < 0.01. C. Competition assay in which binding of sera from hED44Her2-FrC-vaccinated mice to hHer2 on D2F2/E2 cells was detected by FACS in the presence of varying concentrations of trastuzumab or control Mab (rituximab). Mean values with standard deviations from groups of four individual mice are shown. Data are representative of three (A) and (B) or two (C) independent experiments.