Figures & data
Figure 1. Epithelial NF-κB inhibition reduces EGFRL858R-mediated tumorigenesis. (A) Histological scoring of tumor burden, (B) representative H&E-stained lung sections (20x magnification), and (C) representative CT images from EGFRL858R and EGFRL858R DN-IκB mice administered dox for 5 weeks (n = 13–14 mice/group; *p < 0.0001). White asterisk designates the heart in CT images. (D) RT-PCR for EGFRL858R (hEGFR) and DN-IκB transgenes using mRNA isolated from lungs of WT, DN-IκB, EGFRL858R, and EGFRL858R DN-IκB mice administered dox for 5 weeks. (E) Kaplan–Meier survival curve of EGFRL858R and EGFRL858R DN-IκB mice after dox administration (for EGFRL858R n = 19 mice, for EGFRL858R DN-IκB n = 10; log rank test p = 0.0028). (F) Histological scoring of tumor burden in EGFRL858R mice administered dox and treated with BAY 11–7082 or vehicle control (n = 8/group; **p < 0.01).
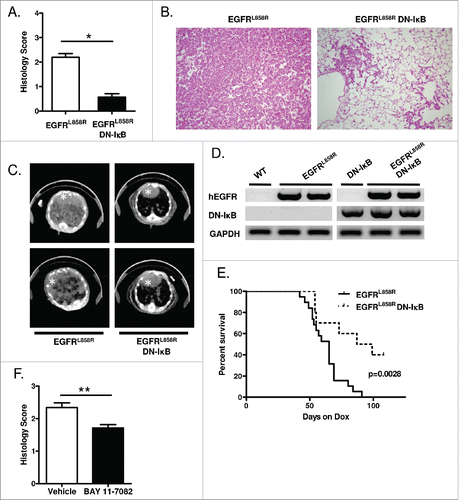
Figure 2. NF-κB inhibition in airway epithelial cells does not alter apoptosis or EGFR-regulated signaling pathways. (A) Western blot of cytoplasmic and nuclear protein extracts from WT and EGFRL858R-mutant immortalized human bronchial epithelial cells transfected with DN-IκB expression vector or control empty vector. β-actin and TATA-binding protein (TBP) were probed as cytoplasmic and nuclear loading controls, respectively. (B) Western blot of EGFR-regulated signaling pathways using protein extracts from WT and EGFRL858R-mutant immortalized human bronchial epithelial cells transfected with DN-IκB expression vector or control empty vector. (C) Western blot and densitometry of EGFR-regulated signaling pathways (D) phospho-Akt, (E) phospho-ERK, and (F) phospho-STAT3 using lung lysates from EGFRL858R and EGFRL858R DN-IκB mice treated with dox for 2 weeks.
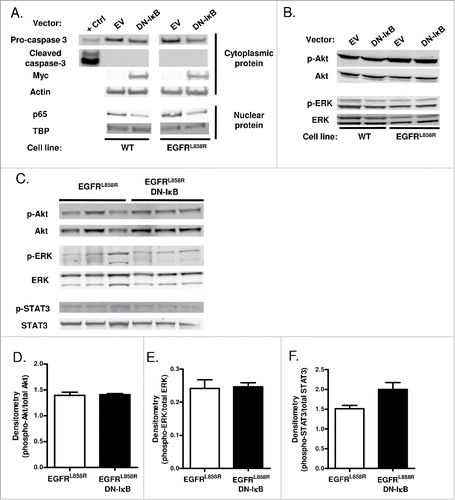
Figure 3. Inhibition of epithelial NF-κB signaling decreases inflammatory cells in the lung during carcinogenesis. (A) Total BAL inflammatory cell numbers from WT, DN-IκB, EGFRL858R, and EGFRL858R DN-IκB mice administered dox for 2 weeks or 5 weeks (for 2 week n = 6–8/group, for 5 week n = 6–14; *p < 0.05, **p < 0.01, ***p < 0.001). (B) Quantitation and (C) representative lung photomicrographs (40x magnification) of CD68 macrophage immunostaining of lung sections from EGFRL858R and EGFRL858R DN-IκB mice administered dox for 2 weeks or 5 weeks (for 2 week n = 4/group, for 5 week n = 5/group; **p < 0.01). (D) Flow cytometry analysis of Ly6G+ cells from CD11b+CD45+ cells in lung single cell suspensions from WT, DN-IκB, EGFRL858R, and EGFRL858R DN-IκB mice administered dox for 2 weeks or 5 weeks (for 2 week n = 3–4/group; for 5 week n = 2–5/group; *p < 0.05). (E) Flow cytometry analysis for CD4+ and CD8+ T cells using lung single cell suspensions from WT, DN-IκB, EGFRL858R and EGFRL858R DN-IκB mice administered dox for 2 weeks (n = 3–4/group).
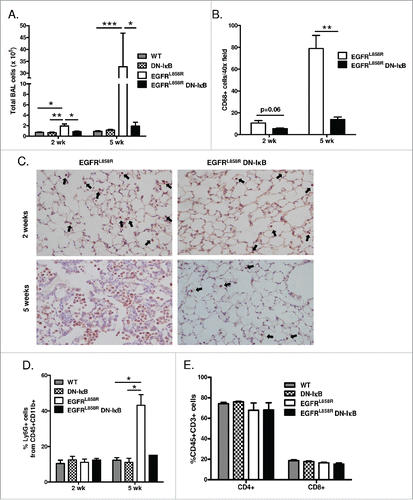
Figure 4. NF-κB inhibition reduces tumor burden and inflammatory cells in the TKI-resistant EGFRL858R+T790M lung carcinogenesis model. (A) Histological quantitation of lung tumor burden and (B) representative images from H&E-stained lung sections (10x magnification) from EGFRL/T and EGFRL/T DN-IκB mice treated with dox for 8 weeks (n = 16 mice/group; ***p < 0.001). (C) RT-PCR for EGFRL/T transgene (hEGFR) expression using mRNA isolated from lungs of EGFRL/T and EGFRL/T DN-IκB mice treated with dox for 8 weeks. (D) Total BAL inflammatory cells and (E) differential cell counts from EGFRL/T and EGFRL/T DN-IκB mice treated with dox for 8 weeks (n = 16 mice/group; **p < 0.01, *p < 0.05). (F) Quantification and (G) representative lung photomicrographs (40x magnification) of CD68 macrophage immunostaining of lung sections from EGFRL/T and EGFRL/T DN-IκB mice treated with dox for 8 weeks (n = 6–8/group; **p < 0.01).
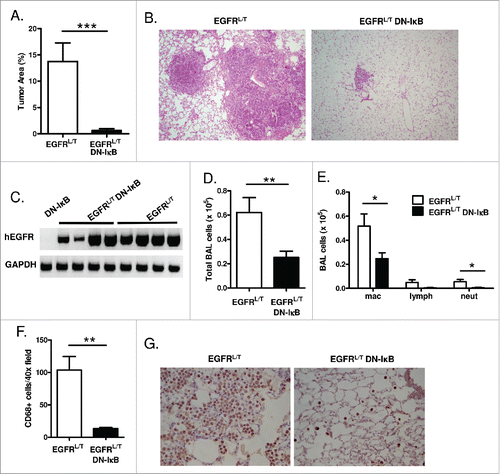
Figure 5. NF-κB signaling promotes macrophage and neutrophil migration. (A) In vitro Boyden chamber chemotaxis assay for neutrophils using BAL fluid from EGFRL858R and EGFRL858R DN-IκB mice treated with dox for 5 weeks (n = 8/group; ***p < 0.001). (B) Quantitative real-time PCR for KC and MIP-2 using mRNA from lungs of EGFRL858R and EGFRL858R DN-IκB mice administered dox for 5 weeks (n = 5–9/group; *p < 0.05; all EGFRL858R DN-IκB MIP-2 measurements below threshold). (C) KC and MIP-2 ELISA measurements using BAL fluid from WT, DN-IκB, EGFRL858R, and EGFRL858R DN-IκB mice treated with dox for 5 weeks (n = 4–10 mice/group; *p < 0.05, **p < 0.01, ***p < 0.001). (D) Boyden chamber chemotaxis assay for macrophages using lung lysates from EGFRL858R and EGFRL858R DN-IκB mice treated with dox for 5 weeks (n = 5–8/group; **p < 0.01). (E) Quantitative real-time PCR for CCL2 and M-CSF using mRNA from lungs of EGFRL858R and EGFRL858R DN-IκB mice treated with dox for 2 weeks (n = 4/group). (F) Protein expression array for inflammatory mediators using whole lung lysates from EGFRL858R and EGFRL858R DN-IκB mice treated with dox for 2 weeks.
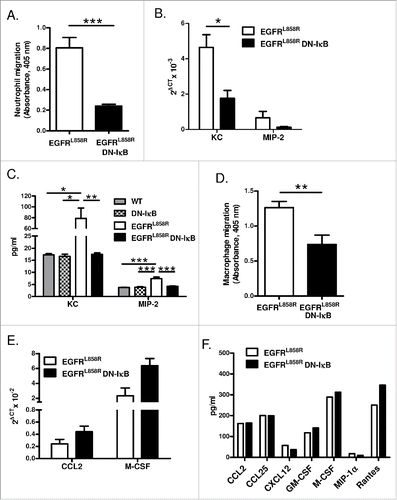
Figure 6. Macrophages promote EGFR-mediated lung tumorigenesis. (A) Flow cytometry analysis of lung single cell suspensions (n = 4/group; *p < 0.05), (B) quantification of neutrophils as a percentage of total white blood cells in peripheral blood smears (n = 7/group; **p < 0.01), and (C) histological analysis of tumors (n = 11–14/group) from EGFRL858R mice administered dox for 5 weeks and treated with Ly6G depletion or IgG isotype control antibodies. (D) Quantification of CD68 macrophage immunostaining (n = 3–5/group; *p < 0.05) and (E) histological analysis of tumors (n = 12–14/group; **p < 0.01) on lung sections from EGFRL858R mice administered dox for 5 weeks and treated with weekly IT injections of PBS or clodronate liposomes.
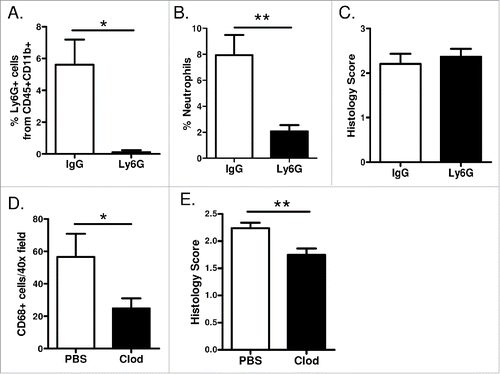
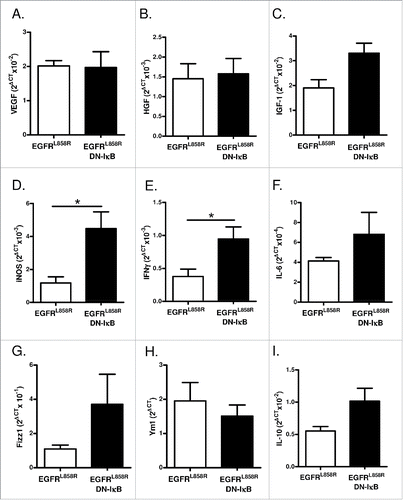
Figure 7. Macrophages from EGFRL858R DN-IκB mice have increased expression of M1 markers. Quantitative real-time PCR analysis for (A) VEGF, (B) HGF, (C) IGF-1, (D) iNOS, (E) IFNγ, (F) IL-6, (G) Fizz1, (H) Ym1, and (I) IL-10 expression in macrophage-enriched cell population isolated from EGFRL858R and EGFRL858R DN-IκB mice treated with dox for 2 weeks (n = 4/group). *p < 0.05.