Figures & data
Figure 1. Chi-Tn mAb specifically binds Tn expressed at different levels on various tumor cell lines. (A) Jurkat, Shin-3, OvCar-3 or TA3Ha cells were labeled with the Chi-Tn mAb or a control antibody (IvIg for human cells or trastuzumab for murine cells) at 20 µg/mL, then with a GaH-Fc-PE secondary antibody. DAPI-negative living cells were acquired by flow cytometry and the PE mean fluorescence intensity (M.F.I.) was determined and is indicated for each sample. Numbers in the quadrants: % of cells. (B) The number of Tn motifs expressed at the cell surface of different tumor cell lines was estimated by quantitative flow cytometry using a murine anti-Tn mAb.
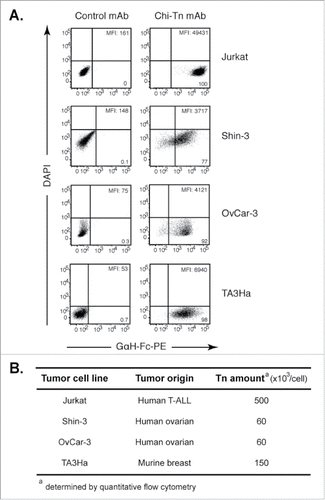
Figure 2. In vivo biodistribution of the Chi-Tn mAb. (A) Nude mice were grafted s.c. with 4 × 106 Shin-3 tumor cells, and were injected i.p. on day 12 with the Chi-Tn mAb or the control mAb at 20 mg/kg. On day 14, solid tumor and organs were removed and sectioned for immunofluorescence (IF) studies. (B) To detect Tn-positive cells, tissue sections were labeled with the Chi-Tn mAb, and with GaH-Fc-Biot and Sa-Cy3. (C) and (D) To detect the presence of the Chi-Tn mAb (or control mAb, red), tumor (C) or tissues (D) sections were labeled directly with GaH-Fc-Biot and Sa-Cy3. Nuclei were labeled using DAPI (blue), and images were acquired by microscopy.
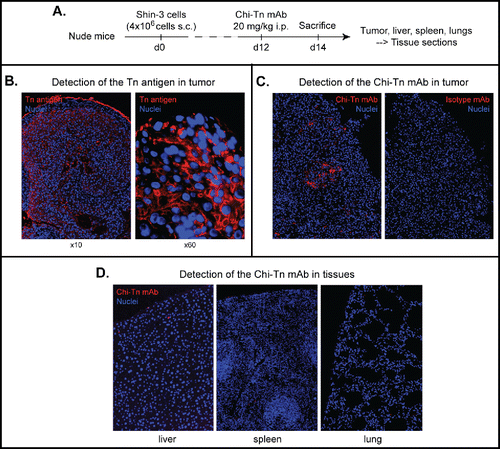
Figure 3. The Chi-Tn mAb is internalized into cancer cells. (A) Jurkat, Shin-3 or TA3Ha cells were incubated for 15 min on ice with the Chi-Tn mAb or with a control antibody (IvIg for human cells or trastuzumab for murine cells) at 20 µg/mL, washed, then transferred to 37°C for the indicated times. Cells were then labeled with GaH-Fc-PE, and the Chi-Tn mAb M.F.I. was determined by flow cytometry in the DAPI-negative living cells gate. For each sample, the Chi-Tn M.F.I. is expressed as a percentage of the Chi-Tn M.F.I. obtained for cells not transferred to 37°C (control cells). (B) Jurkat, OvCar-3, Shin-3 or TA3Ha cells were incubated on ice with the Chi-Tn mAb for 15 min, washed and transferred to 37°C for the indicated period of time. Cells were then fixed to glass coverslips and labeled. Yellow: actin network; pink: membrane-bound or internalized Chi-Tn mAb; blue: DAPI. Arrows indicate examples of internalized Chi-Tn mAb.
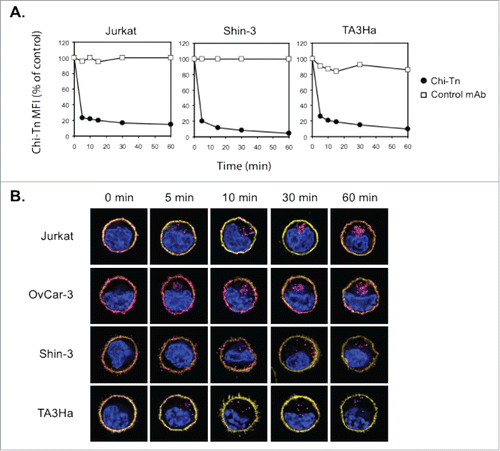
Figure 4. Analysis of the Chi-Tn mAb targeting to endosomal compartments Jurkat cells were labeled with the Chi-Tn mAb, transferred to 37°C for the indicated times, then actin (yellow) and Chi-Tn (pink) were detected in fixed cells. Early endosomes were detected using Tf-A488 (green, A), recycling endosomes with Rab11 (green, B), and late endosomes with anti-LAMP-1 mAb (green, C). Blue: DAPI. Images were acquired by deconvolution 3D-microscopy. Examples of co-localization (in white) are indicated by arrows.
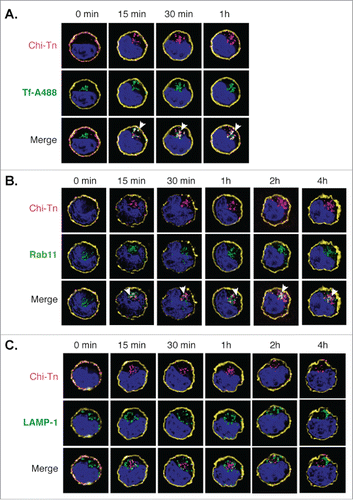
Figure 5. In vitro and in vivo cytotoxicity of Chi-Tn-ADC on cancer cells (A–D) In vitro cytotoxicity of Chi-Tn ADC. (A) TA3Ha, Jurkat, OvCar-3 or Shin-3 cells were cultured with the indicated concentrations of free saporin (SAP). (B) Jurkat, TA3Ha (left panel), and OvCar-3 and Shin-3 cells (right panel) were cultured with the indicated concentrations of Chi-Tn mAb (open symbol) or the Chi-Tn/SAP conjugate (filled symbol). (C) Jurkat and SKBR3 cells were cultured with free MMAF. (D) Jurkat (left panel) and SKBR3 (right panel) cells were cultured with Chi-Tn/MMAF or Her/MMAF conjugate, naked Chi-Tn or Her mAb. Cell viability was assessed after 3 d of culture. Results are expressed as percentage of viable cells compared to untreated cells. (E–H) In vivo cytotoxicity of Chi-Tn/MMAF against Tn+ LOX tumor cells. (E) LOX cells express a high and stable level of Tn. LOX cells were labeled with the Chi-Tn mAb or a control antibody at 20 µg/mL, then with a GaH-Fc-PE secondary antibody and Tn expression was measured by flow cytometry. (F) LOX cells were cultured with the Chi-Tn/MMAF or Her/MMAF conjugate at the indicated concentrations. Cell viability was then assessed after 3 d at 37°C. Results are expressed as percentage of viable cells compared to untreated cells. (G) Experimental schedule of the in vivo antitumor assay. Nude mice were grafted with the LOX Tn+ human cell line and then treated twice a week with the Chi-Tn/MMAF (n = 8) or control Her/MMAF (n = 6) conjugates, with the naked Chi-Tn mAb (n = 6), or were left untreated (n = 7). (H) Tumor growth is depicted as Relative Tumor Volume (RTV), as explained in the Materials and Methods section. Statistical significance was calculated with Mann-Whitney test; significant statistical difference was observed on days 20 and 25 between Her-MMAF and Chi-Tn-MMAF treated groups (*p < 0,05).
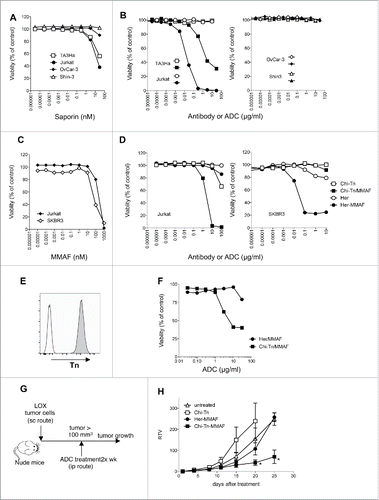
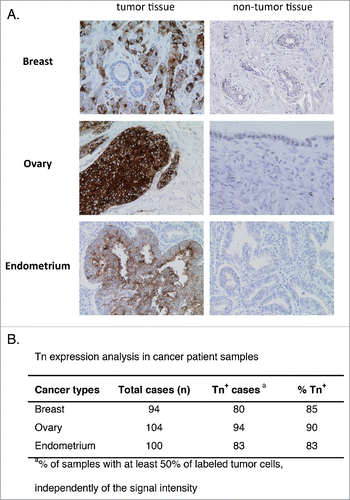
Figure 6. Analysis of Tn expression in cancer samples from patients. Breast, ovary, and endometrium tumor samples were analyzed by IHC. (A) Representative images of Tn expression in tumor and in healthy breast, ovary and endometrium tissues. (B) Quantification of Tn expression in the different tumor samples.